Cellular and molecular biology of small cell lung cancer: an overview
Introduction
Small cell lung cancer (SCLC) is the most aggressive subtype of lung cancer, characterized by a 5-year survival rate of <7%. Considering that standard treatments have not changed in decades, a deeper understanding of cellular and molecular mechanisms underlying SCLC initiation, progression, metastasis and acquisition of resistance is required to improve the available treatment options. In this review, we focus on the biology of SCLC, including histopathological, molecular and immuno-related factors that can give us some new ideas and paths in order to develop newer more effective treatments.
Cell of origin of SCLC
The lung epithelium contains several distinct stem cell and progenitor cell populations that maintain the numerous types of differentiated lung cell populations (1-4). Mucous, basal, ciliated, non-ciliated (Clara and serous) and neuroendocrine cells (NECs) line the conducting airways of the lung. Alveolar type 1 (AT1) and type 2 (AT2) epithelial cells line the alveolar space, secrete surfactants and perform gas exchange, respectively. A population of presumptive distal adult lung epithelial stem cells [bronchioalveolar stem cells (BASCs)] was also recently identified at the bronchioalveolar duct junction (BADJ) (5) and reported as being the origin of non-small cell lung cancer (NSCLC). Previous work shows that NSCLC may originate from BASCs (6).
SCLC has a very high mutation load due to the long-term exposure to carcinogens present in cigarette smoke (7). SCLC cells have a high mitotic index, display neuroendocrine markers and are believed to derive from NECs or neuroendocrine progenitors (NEPs) in the lung. However, the cell of origin of SCLC has not been formally identified. Mouse models replicating the oncogenic mutations and tumor suppressor losses observed in patients have pointed to pulmonary NECs, or a multipotential precursor that gives rise to pulmonary NECs, as the cell of origin for SCLC (8-10). Human and mouse SCLCs predominately localize to the midlevel bronchioles and typically express a range of neuroendocrine markers, such as calcitonin-gene related peptide (CGRP) and neural cell adhesion molecule (NCAM1). These SCLCs also express transcription factors which play important roles in neuroendocrine differentiation, including achaete-scute complex homolog-like 1 (ASCL1; murine ortholog Mash1), NEUROD1, SOX2, TTF1, and Myc family members (11). ASCL1 is a basic-helix-loop-helix transcription factor pivotal for neuroendocrine differentiation and expressed in pulmonary NECs and in SCLC (12). ASCL1 may interact with the central “retinoblastoma protein-tumor protein 53” (Rb-p53) axis in the carcinogenesis of NE lung cancers (13) and ASCL1 expression correlates with the tumor-initiating capacity of SCLC tumors (14). ASCL1 contributes to enhanced proliferation and migration in lung cancer cells in vitro by targeting cyclin-dependent kinase 5 (CDK5) (15) and is critical for pulmonary NEC development (12,16-18). ASCL1 is also important in NEC fate and is highly expressed in classic SCLC and large cell neuroendocrine carcinoma (LCNEC) tumors, where it acts to maintain neuroendocrine features (12). Using next generation sequencing and establishing features of ‘small cell-ness’, Meder et al. identified a NOTCH-ASCL1-Rb1-TP53 signaling axis which may drive SCLC in organs other than the lung (19). The expression of ASCL1 and NEUROD1, another transcription factor, are mutually exclusive in SCLC cell lines. ASCL1 but not NEUROD1 is required for neuroendocrine tumor (NET) formation in the Trp53/Rb1/p130 mouse model (20). Studies have indicated that extracellular signal-regulated kinases (ERK) 1/2 signaling is low in all SCLC cell lines and that blocking ERK activity has no effect on cell growth while activating ERK inhibits SCLC growth. NEUROD1 can inhibit ERK and stimulates metastases (21,22). NEUROD1 binds the TrkB promoter and inhibition of TrkB by chemical means blocks SCLC proliferation in vitro and in vivo (22).
Extensive genomic analyses have been carried out and there is a panel of several genes that appear mutated/abnormal in nearly all SCLCs, such as p53, Rb and Myc family members. In addition, certain oncogene mutations such as KRAS are rare or non-existent. Predominant mutations observed in SCLC include loss-of-function mutations in the Rb and p53 genes. Overexpression of proto-oncogenes by amplification of distinct chromosomal regions include L-myc or C-myc (23,24). Since mutation rate patterns can distinguish the cell-of-origin (25) this result suggests that SCLC and adenocarcinoma have a different progenitor, a finding that is in line with a recent study on transgenic SCLC mouse models (10). Berns and colleagues have described a mouse model of SCLC based on the fact that tumor cells in more than 90% of human SCLCs are mutated for both the p53 and Rb tumor suppressor genes (11,26). In this model, adenoviral particles expressing the Cre recombinase (Ad-Cre) were injected into the trachea of p53 and Rb1 mutant genetically engineered mouse models (GEMM) to delete both genes in neuroendocrine (CGRP promoter), Clara (Clara Cell marker 10; CC10 promoter) but also AT2 (Surfactant protein C; SPC promoter) cells. Nearly all of these Rb/p53 double mutant mice develop SCLCs, although it takes often more than nine months before tumors become evident. The tumors closely resemble human SCLC, express markers of NECs and even metastasize to the same organs (11). They also acquire additional mutations that are reminiscent of human SCLC, such as the amplification of one of the Myc genes (11,27). However, also switching off Rb and p53 in non-NECs SPC-positive cells gives rise to SCLC, although less efficiently. Interestingly, the latter tumors are often more peripherally located, and preliminary data suggest they might carry chromosomal aberrations that are not observed in tumors induced in NECs (8,10). For instance, amplification of the transcription factor nuclear factor I/B (NFIB) both in a genetically-engineered mouse SCLC model and in human SCLC has a synergistic effect with L-myc, and functional studies indicate that NFIB regulates cell viability and proliferation during transformation (28,29). It is also unclear whether the same cell of origin is responsible for initiating both SCLC and NSCLC. The observation that a proportion of SCLCs display a mixture of SCLC- and NSCLC-specific features may argue for the existence of a “common” cell of origin for these lung cancers (30).
Histological features of SCLC and differential diagnosis. Typical and atypical carcinoid-large cell neuroendocrine cancers
SCLC is located along the spectrum of NET of the lung, being a high-grade tumor. Also on this spectrum are LCNEC, a low-grade typical carcinoids (TC) and intermediate-grade atypical carcinoids (AC) (31). While the aetiology is still not completely understood, carcinoids appear to arise from a different progenitor cell than SCLC and LCNEC. The first clue to this difference is a possible pre-neoplastic lesion “diffuse idioplastic neuroendocrine cell hyperplasia” (DIPNECH), unique to carcinoids and their occurrence in the setting of MEN1 disease (5% of carcinoids arise in MEN1 disease and less than 5% in the background of DIPNECH). Twenty to forty percent of carcinoids display somatic double allelic inactivation of MEN1 gene with mutation of one allele and allele loss (LOH) at the MEN1 gene (11q.13) location (32). A very original mutation profile of carcinoids has been identified with 52% of the mutations affecting chromatin remodelling genes (MEN1, PSIP1, ARID1A), 34% belonging to the methylation complex and 25% to the SWI/SNF complex, mutually exclusive to each other (33). In contrast, a close biologic relationship between LCNEC and SCLC has been suggested (34), although whether LCNEC should be therapeutically treated as SCLC or NSCLC remains controversial (35,36). Still, it seems that LCNEC may be comprised of distinct molecular subsets including SCLC-like, characterized by concomitant loss of RB1 and TP53, and NSCLC-like, characterized by the presence of NSCLC-type mutations, including KRAS, STK11, KEAP1 or MAP2K1 (37).
The main criteria for distinguishing SCLC from TC, AC and LCNEC are summarized in Table 1.
The proliferation rate as defined by Ki-67 staining can also be useful as it is very high in SCLC (usually >50–70%), but low (usually <5–15%) in carcinoids. However, precise Ki-67 thresholds for TC vs. AC are not established (39).
Histological sub-classification of SCLC has advanced significantly since 1962 when Kreyberg proposed the oat cell and polygonal cell types (40). The 1981 WHO classification, which defines an “intermediate” subtype of SCLC, was not successful because clinicians were never sure whether these subtypes were SCLC or NSCLC. In addition, reproducibility was poor among expert pathologists and it was not clear whether there were any relevant clinical implications (41). The current sub-classification recognizes only two subtypes: pure SCLC and combined SCLC (38). Using light microscopy, tumor cells have round, spindled nuclei with finely granulated chromatin, inconspicuous nucleoli, scant cytoplasm, and frequently show nuclear moulding (38). Mitotic rates are high, with an average of 80 mitoses per 2 mm2, and necrosis is frequent and often extensive (38,42,43). Basophilic encrustation of vessel walls by DNA from necrotic tumor cells (nuclear debris also known as the Azzopardi effect) is often seen in necrotic areas (44). The pattern of growth, which usually occurs in diffuse sheets, rosettes, organoid nesting, streams and ribbons may resemble malignant lymphoma (42).
Although immunohistochemistry (IHC) is useful in the diagnosis of SCLC, it is only required in problematic cases, since it is important to obtain a good-quality hematoxylin and eosin stain. Staining for pancytokeratin helps to demonstrate whether the tumor is a carcinoma rather than lymphoid lesion. CK7 and CK20 are not particularly useful cytokeratins for SCLC diagnosis since only about half stain with CK7 and <10% with CK20 (45). The most useful neuroendocrine markers include CD56, chromogranin and synaptophysin (42). Neuroendocrine marker staining may either be diffuse and strong for all three neuroendocrine markers, or focal or weak with only one or two positive markers. In <10% of cases, all neuroendocrine markers may be negative but the diagnosis can still be established based on the morphological context (46). CD56 stains approximately 90–100% of SCLCs but with low specificity (47). Almost two-thirds of SCLC will be negative for chromogranin and synaptophysin (48). The thyroid transcription factor 1 (TTF-1) expression is present in 70–90% of SCLC cases but can also be positive in 44–80% of extrapulmonary small cell carcinomas and therefore is not useful in determining the primary site of SCLCs (49). In cases in which all neuroendocrine markers and TTF-1 are negative, lymphoma, melanoma and basaloid carcinoma with positive keratin expression should be excluded, as should negative squamous markers such as p63 (46). It is worth mentioning here that complete phenotypic conversion to SCLC is one possible mechanism of resistance in NSCLC adenocarcinomas progressing on epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) treatment and occurs in roughly 15% of patients (50,51). These tumors usually retain the original EGFR mutation, arguing against them being newly generated primary tumors, but can continue to proliferate malignantly despite downregulation of EGFR protein, explaining their resistance to TKIs.
Molecular abnormalities in SCLC—the tumor suppressor role of the NOTCH pathway
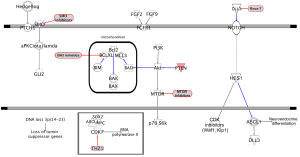
Almost universal and bi-allelic loss of TP53 and RB1 occurs in SCLC (52,53). Even tumors lacking RB1 mutations show evidence of chromothripsis leading to overexpression of cyclin D1 (encoded by the CCND1 gene), revealing an alternative mechanism of RB1 deregulation (54). PTEN alterations can be found in 10–18% of SCLC tumors (55), while deregulation of Myc function has also been seen to be important (24). Although NOTCH pathway activation acts as an oncogenic stimulus in some tumor types (56), NOTCH activation in NET suppresses tumor growth (57). Whole-genome sequencing of 110 human SCLC specimens identified, as one of the hallmarks of SCLC, inactivating mutations of NOTCH family genes in about 25% of human SCLC tumors, suggesting these genes are tumor suppressors in SCLC. This notion was further supported by the observation that activation of NOTCH signaling leads to significantly fewer tumors and prolonged survival in SCLC transgenic mouse models (54). A characteristic of primary SCLC is high-level expression of ASCL1. During lung cancer development, NOTCH1 and HES1 are highly expressed in non-NE airway epithelial cells, whereas ASCL1 expression is restricted to clusters of pulmonary NECs. NOTCH seems to control pulmonary-epithelial-cell fate by activating HES genes, which suppress the NEC fate by repressing ASCL1 (12,58). HES1 negatively regulates ASCL1 by binding to and thereby repressing its promoter. Interestingly, NOTCH1-IC (intracellular domain) and NOTCH2-IC can cause cell-cycle arrest in a number of SCLC-derived cell lines with neuroendocrine differentiation by upregulating CDK inhibitors p21 (Waf1) and p27 (Kip1) and negatively regulating ASCL1 (59,60) (Figure 1). The mammalian NOTCH family ligands delta-like 1 (DLL1), DLL4, JAG1, and JAG2 each activate NOTCH receptor signaling in trans (61). In contrast, the related ligand DLL3 predominantly localizes to the Golgi apparatus and is unable to activate NOTCH signaling (62,63). In normal development, DLL3 inhibits NOTCH pathway activation by interacting with NOTCH and DLL1 and redirecting or retaining them to late endosomal/lysosomal compartments or the Golgi respectively, thereby preventing their localization to the cell surface (62,64). DLL3 is one of several NOTCH ligands that appear to be direct downstream targets of ASCL1 (20,65) (Figure 1). Therefore DLL3 might be associated with the neuroendocrine phenotype and contributes to neuroendocrine tumorigenesis. Indeed, Saunders et al. demonstrated that DLL3 is associated with the neuroendocrine cancer phenotype (66). In vivo efficacy of a DLL3-targeted antibody-drug conjugate (ADC), SC16LD6.5, comprised of a humanized anti-DLL3 monoclonal antibody conjugated to a DNA-damaging pyrrolobenzodiazepine (PBD) dimer toxin, correlated with DLL3 expression. Responses were observed in patient-derived xenograft (PDX) models from both limited and extensive-stage disease and were independent of their sensitivity to standard-of-care chemotherapy regimens (66). Interestingly, this year at the European Society for Medical Oncology (ESMO) meeting, Pietanza et al. reported the results of a phase I trial of rovalpituzumab tesirine (Rova-T, or S16LD6.5), in 79 patients with SCLC who had progressed after first or second line therapy. High expression of DLL3 occurred in approximately two-thirds of SCLC patients and was related to long lasting stable disease or partial response to Rova-T (67).
Epigenetic and proteomic changes in SCLC—the role of the DNA repair
A primary mechanism of epigenetic regulation of gene expression is DNA methylation. Hypermethylation of CpG islands associated with regulatory elements controlling gene expression can drive secondary recruitment of histone modifications, together leading to stable gene silencing. SCLC is characterized by extreme plasticity and cloning capacity consistent with a high level of stemness (68).
Using methylome and transcriptome analysis, distinct subtypes of SCLC can be defined that cannot be distinguished by standard histological approaches (69). Indeed, epigenetically distinct subgroups were observed among histologically and genetically similar SCLC cases that may represent clinically important populations. These subgroups have widely differing transcriptional profiles and can be delineated by differential expression of the neurogenic basic helix-loop-helix transcription factors ASCL1 and NEUROD1, suggesting that primary SCLC can be found along the same spectrum of differentiation as in cell lines and PDX (70).
Enhancer of zeste homolog 2 (EZH2) showed increased overexpression in comparison to normal lung tissue, correlated with a higher methylation of the EZH2 promoter (70). Activation of the oncogene EZH2 is a major consequence of RB/E2F pathway deregulation and EZH2 expression has a significant effect on SCLC viability. EZH2 promotes the cell cycle and inhibits apoptosis in SCLC, favoring the oncogenic processes of the RB/E2F pathway (52,71). By profiling DNA methylation in SCLC, PDXs and cell lines at single-nucleotide resolution, it has been found that DNA methylation patterns of primary samples are distinct from those of cell lines, whereas PDX maintains a pattern closely consistent with primary samples (70). Clustering of DNA methylation and gene expression of primary SCLC revealed distinct disease subtypes among histologically indistinguishable primary patient samples with similar genetic alterations. Pharmacologic inhibition of EZH2 in a SCLC PDX markedly inhibited tumor growth (70). Recent developments in drug discovery (72,73) and pre-clinical data suggest that EZH2 is sensitive to targeted therapy.
Proteomic analysis on SCLC tumors has shown that Poly ADP ribose polymerase 1 (PARP1) inhibition has activity in pre-clinical models and in a subset of SCLC patients (74) and proteomic markers of DNA repair and phosphatidylinositol 3-kinase (PI3K) pathway activation are predictive of response to PARP inhibition in SCLC (75). Significant differences in signaling pathways have been reported between SCLC and NSCLC, with PARP1 and EZH2 the most overexpressed proteins in SCLC (76,77). PARP inhibition downregulates key components of the homologous recombination pathway, such as RAD51 and BRCA1. Due to the loss of RB, E2F expression leads to activation of several E2F targets in SCLC cells, such as EZH2, thymidylate synthase (TS), and several components of DNA repair pathways, including 53BP1 (76,78). Single agent activity of the PARP inhibitor ABT-888 (veliparib) and synergy with platinum and etoposide was demonstrated in cell lines and animal models (79). This work led to a successfully completed phase I trial combining veliparib with cisplatin and etoposide (ECOG-ACRIN E2518) (80); a randomized phase II trial is currently ongoing. The newer PARP inhibitor talazoparib kills SCLC cells more efficiently than the older PARP inhibitor olaparib (75) and high levels of PARP and other proteins involved in DNA damage repair, such as FANCD2 and pCHK2, are strongly associated with the sensitivity of SCLC cells to talazoparib. High expression of a “DNA repair protein score” is also associated with greater response to talazoparib, while higher expression of a “PI3K score” denotes grater resistance to the drug (81).
New SCLC subsets and targets
Although SCLC is characterized by a high mutation rate, usually it harbors loss of function mutations or deletions in tumor suppressor genes and is not susceptible to selective targeted inhibition (54). Emerging data on SCLC biology has led to the discovery of novel biomarkers which are potentially helpful to define molecularly selected subpopulations of patients more likely to benefit from a molecularly targeted approach.
The hedgehog signaling pathway
Hedgehog signaling plays a crucial cell-intrinsic role in the development and maintenance of SCLC, and Hedgehog pathway inhibition can be a therapeutic strategy to slow disease progression of disease and delay cancer recurrence (82). Constitutive activation of the hedgehog signaling molecule smoothened (Smo) promotes the clonogenicity of human SCLC in vitro and the initiation and progression of mouse SCLC in vivo. Reciprocally, deletion of Smo in RB1 and Trp53-mutant lung epithelial cells strongly suppresses SCLC initiation and progression in mice (82). Pharmacological blockade of Hedgehog signaling was found to inhibit the growth of mouse and human SCLC, most notably following chemotherapy (Figure 1).
Fibroblast growth factor receptor (FGFR) signaling pathway
The FGFR family represents a promising target for development of targeted therapies in SCLC. Twenty-three fibroblast growth factors (FGF) and four FGFRs (FGFR1–FGFR4) have been identified (83) and results of several studies have demonstrated the co-expression of FGF2 and FGF9 ligands in association with FGFR1 in human lung cancers (84). The FGFR1 gene can be amplified in 5–6% of SCLC patients (85). Tumor tissue from 63 SCLC patients treated with surgery was sequenced, and amplification of FGFR1 was observed in 6% of the cases (52). High levels of serum FGF2 have been associated with poor prognosis in SCLC, possibly due to a FGF2-mediated cytoprotective effect, whereby the expression of antiapoptotic proteins is upregulated, promoting resistance to current anticancer treatment (86) (Figure 1). FGFR1, FGF2 and FGF9 protein and messenger RNA (mRNA) expression, as well as FGFR1 gene amplification were analyzed in primary tumors from 90 SCLC patients. FGFR1 protein expression was identified in 7% of cases and was significantly correlated with FGFR1 mRNA levels, FGFR1 gene amplification and FGF2 and FGF9 protein expression (87). Therefore, combined analysis of FGFR1 and ligand expression may allow selection of SCLC patients for FGFR1 inhibitor therapy. The efficacy of the FGFR inhibitor ponatinib is currently being studied in a biomarker driven trial (NCT01935336). Another phase II trial to assess the efficacy and safety of lucitanib, an inhibitor of FGFR1–3, vascular endothelial growth factor receptor (VEGFR)1–3, and PDGFRα/β, in patients with advanced lung cancer is currently recruiting (NCT02109016).
Rearranged during transfection (RET) pathway
RET is a transmembrane tyrosine kinase receptor located in the pericentromeric region of the chromosome 10q11.2 and involved in the physiologic development of kidney, nervous and neuroendocrine systems (88). Binding with soluble ligands belonging to glial cell line-derived neurotrophic factor (GDNF) family induces dimerization and autophosphorilation of the intracellular tyrosine residues of RET receptor, mediating pathway activation (89). An activating somatic mutation of RET (M918T) has been identified in the brain metastatic lesion of a patient with SCLC. In SCLC cell lines, the presence of the M918T mutation determines the downstream activation of Myc and ERK regardless of the presence of a GDNF ligand (90). Moreover, SCLC cell lines harboring RET M918T show a high proliferation rate compared to RET wild type cell lines, suggesting that RET may act as an oncogene during SCLC development. Unlike medullary thyroid carcinomas with MEN2B syndrome that harbor RET M918T in the 95% of cases, the presence of this alteration seems to be a rare occurrence in SCLC.
PI3K, AKT, and mammalian target of rapamycin (mTOR) pathway—the role of intrinsic apoptosis
The PI3K/AKT/mTOR pathway is a prototypic survival pathway constitutively activated in many types of cancer (Figure 1). Drug resistance in SCLC may be attributable to the persistence of a subpopulation of cancer stem-like cells (CSC) that exhibit multiple drug resistance (91). VS-5584 is a potent and selective dual inhibitor of mTORC1/2 and class I PI3-kinases currently in early phase clinical trials that has been proposed for targeting CSCs (92).
In a Japanese population, genetic alterations in the PI3K/AKT/mTOR pathway have been detected in 36% of SCLC tumors (93). In H466 (with PTEN loss) and H1048 (with PIK3CA mutation) cell lines, high expression of phosphorylated AKT and S6K was observed. Moreover, silencing PIK3CA in H1048 cells through small interfering RNA (siRNA) or using drugs (BEZ235, BKM120, INK128, MK2206) targeting the PI3KCA/AKT/mTOR pathway significantly reduced cell proliferation, suggesting the strong dependence of these cells from the dysregulated PI3KCA/AKT/mTOR pathway (93). To date, only limited evidence of activity is available with single mTOR inhibitors in SCLC. Nevertheless, a phase I study with BEZ235, a dual PI3K and mTORC1/2 inhibitor, is currently ongoing (NCT01195376). In addition, there is evidence that the combination of BH3 mimetics with mTOR inhibitors can be a potent combination therapy for SCLC. Although the anti-apoptotic protein Bcl-2 is overexpressed in many cancers, including 40–60% of SCLCs and the BH3 mimetic navitoclax (ABT-236) promotes apoptosis in SCLC cell lines (94-96), early phase clinical trials failed to demonstrate any significant clinical benefit (97,98). Faber et al. found that although SCLC cell lines have higher expression levels of Bcl2-interacting mediator of cell death (BIM), which can “prime” tumors for apoptosis and contribute to the sensitivity to ABT-263, the lack of sensitivity of SCLC to ABT-263 in the clinic may be due to high levels of the prosurvival Bcl-2 family member myeloid cell leukemia 1 (MCL-1) in these cancers (99) (Figure 1). SCLC can be sensitized to ABT-263 via TORC1/2 inhibition, leading to reduction of MCL-1 protein levels and thereby permitting BIM-mediated apoptosis (99). The BIM to MCL-1 ratio predicted sensitivity to ABT-263 not only in SCLC, but also across a large panel of cancer cells encompassing a wide range of malignancies (99). This suggests that dual inhibition of these pathways might be a rational therapeutic approach in SCLC.
Transcriptional addictions of SCLC
CDK7 is involved in transcription initiation by phosphorylating the Ser5 residue of the RNA polymerase II C-terminal domain at gene promoters. Using high throughput cellular screening of a chemical library, Christensen et al. observed that SCLC cell lines and in vivo models are highly sensitive to THZ1, a covalent irreversible inhibitor of CDK7 (100) (Figure 1). Expression of super-enhancer-associated transcription factor genes (genes associated with unusually large transcriptional regulatory domains known as super-enhancers), including Myc family proto-oncogenes, neuroendocrine lineage-specific factors, or sex determining region Y-box 2 (SOX2) are highly sensitive to THZ1 treatment (100). Hence, further pharmacological studies of small molecules targeting the general transcription apparatus should be performed to advance the field of SCLC-tailored therapeutics.
The role of immunotherapy in SCLC
The recent successes obtained with immunotherapy in several types of tumors have renewed hopes for treatment of SCLC, despite the limited evidences of activity to date in this setting (101-103). The onset of neurologic paraneoplastic syndromes in a subset of patients with SCLC is related to the T-cell response to onconeural antigens expressed by the tumor (104). For example, patients with the Hu paraneoplastic syndrome typically harbor SCLCs that are limited to single nodules and have improved response to therapy and prolonged survival (105,106). Also, presence of activated effector T-cells in SCLC has been associated with cytolytic responses. In this regard, Koyama and colleagues analyzed the T-cell population in extensive or limited SCLC patients and healthy volunteers. Effector T-cells were found to be higher in those patients with limited stage disease and long-term survival, and lower in patients with recurrent disease. Therefore, for immunotherapy to enhance an effective anti-tumor immune response, effector T-cells must be increased and the T-regulatory population abrogated (107).
Targeting cytotoxic T-lymphocyte antigen 4 (CTLA-4) and PD-1 in SCLC
Targeting the T-cell immune checkpoints programmed cell death 1 (PD-1) and CTLA-4 has recently emerged as an effective therapeutic strategy in several tumor types. Scultheis and colleagues evaluated the PD-1 and PD-1 ligand (PD-L1) expression in 94 small cell neuroendocrine carcinomas (61 pulmonary) using IHC and RNA expression. They found that carcinoma cells were negative in all cases, and that PD-L1 is expressed in tumor-infiltrating macrophages and its expression is related to the presence of tumor-infiltrating lymphocytes (108). The pivotal study of Snyder et al., performing whole-exome sequencing of tumor samples from melanoma patients treated with anti-CTLA-4 specific antibodies ipilimumab and pembrolizumab, suggested that high somatic mutation load correlates with response to therapy (109). SCLC has an exceptionally high number of somatic mutations, approximately 30% in protein changing genes, predominantly G to T transversions, related to the carcinogenic effects of tobacco smoke on DNA. The second malignancy with a high mutational burden is melanoma, induced by another potent carcinogen in the form of ultraviolet light (53). Differential activity of immune checkpoint inhibitors according to the smoking status has been widely described in NSCLC and linked to increased mutational burden in smoking-related cancer. In a retrospective analysis of NSCLC patients treated with nivolumab, a monoclonal antibody targeting PD-1, the response rate was significantly higher in former/current smokers compared with minimal/never smokers (≤5 pack-years) (27% vs. 0%; P=0.034) (110). Similarly, in patients with progressive NSCLC treated with pembrolizumab, current or former smokers had a response rate of 22.5%, as compared with 10.3% among never-smokers (111). Rizvi et al. reported that patients with high nonsynonymous mutation burden experienced improved objective response rate (63% vs. 0%), progression-free survival (PFS) (14.5 vs. 3.7 months) and durable clinical benefit (73% vs. 13%) from pembrolizumab, compared with those with low mutation burden. Efficacy was also related to molecular smoking signature, higher neoantigen burden and DNA repair pathway mutations (112). The reliable association between response to immune checkpoint inhibitors in NSCLC, mutation burden and smoking history is strongly applicable to SCLC, which nearly entirely related to smoking and characterized by an elevated mutation burden.
Immune checkpoints inhibitors have been evaluated in SCLC to enhance the anti-cancer effects of T-cells against tumor. In a phase II clinical trial evaluating ipilimumab in combination with standard chemotherapy in extensive stage SCLC patients, phased administration of ipilimumab resulted in improved immune-related PFS (6.4 vs. 5.3 months; P=0.03), immune-related best overall response rate (71% vs. 53%) and overall survival (OS) (12.9 vs. 9.9 months; P=0.13), compared to paclitaxel and carboplatin alone (113). A phase III trial comparing efficacy of platinum/etoposide with or without ipilimumab in first line treatment of SCLC patients, with OS as the primary endpoint, is still ongoing (NCT01450761). Furthermore, blocking PD-L1 signaling by infiltrating macrophages seems to be sufficient to generate a positive response from T-cells and improve survival in SCLC patients (114). Several clinical trials investigating PD-1 or PD-L1 inhibitors in SCLC patients are currently ongoing (e.g., NCT01928394, NCT01693562, NCT02261220, NCT02402920, and NCT02359019).
Vaccine therapies for SCLC
Considering the biological impact of immunity in SCLC, cancer vaccines may theoretically represent a promising approach. Key to obtaining effective vaccines is the identification of the presence of a reliable tumor-associated antigen expressed in a significant proportion of cancer patients and upon which survival of tumor cells depends. Unfortunately, a clinical trial testing the immunologic and clinical effects of a cancer vaccine consisting of dendritic cells (DC) transduced with the full-length wild-type p53 gene in patients with extensive stage SCLC failed to demonstrate a relevant benefit (102). Another potential target for cancer vaccine is represented by gangliosides which are complex glycolipid constituents of the cellular plasma membrane involved in numerous biologic functions (cell-cell recognition, cell matrix attachment and differentiation), predominantly found in the nervous system but also identified on the surface of SCLC cells (115). GD3 is a glycosphingolipid antigen highly expressed in SCLC and rarely in normal tissue (116). A combinatory vaccine composed of BEC2 (a monoclonal antibody which mimics GD3) and Bacillus Calmette-Guerin (BCG) was studied extensively in SCLC (117). Although BEC2/BCG showed promising results in an early clinical trial in SCLC (103), a large randomized phase III trial failed to demonstrate statistically significant differences in median OS in 515 patients with limited SCLC (16.4 months with BSC vs. 14.3 months with BEC2/BCG; HR =1.12; 95% CI: 0.91–1.371) (118). Fucosyl-GM1 (Fuc-GM1) represents another promising target expressed in the majority of SCLC tumors. Despite limited demonstrated activity (119), a high-affinity antibody against this antigen is currently under investigation in a phase I/II clinical trial (BMS-986012, NCT02247349).
Chimeric antigen receptor (CAR) approaches for SCLC therapy
The immune status in SCLC has been demonstrated to have prognostic relevance (107,114,120). However, so far immunological approaches have not been clinically meaningful. If the presence of an immunosuppressive microenvironment limits the endogenous T-cell elicitation, an ex-vivo T-cell culture expansion may be considered. The process involves extraction of T cells from patients, transfection with a gene for CARs that recognizes cancer-specific antigens, and reinfusion of the transfected cells into the patient. Therefore, the modified T-cells can recognize and kill tumor cells that would otherwise escape immune detection. The advantages of the CAR T-cells lie in the fact that they are HLA-independent, do not require stimulation by the host immune system and may be engineered in order to target selectively specific SCLC antigens (121,122).
The potential targets of CAR T-cells in SCLC can be derived from autoantibodies found in patients. NCAM/CD56 in particular represents an attractive target with high expression on the surface of SCLC cells, as well as in neuronal tissue (123). Preliminary preclinical in vitro and in vivo data suggest potent anti-tumor effects of CD56 CAR T-cells in SCLC. Expression of CD56 on other cells, including neural tissue and natural killer (NK) cells, might limit targeting this antigen, but the strategy could be developed with other SCLC-specific cell surface biomarkers as combinatory therapy. Among promising markers, cell-surface CD47 interacts with its receptor on macrophages, SIRPalpha, to inhibit phagocytosis of normal healthy cells. Nevertheless, CD47 is also constitutively upregulated on mouse and human myeloid leukemia in order to evade macrophage killing (124). Increased CD47 expression is an independent factor of poor prognosis in adult patients with acute myeloid leukemia (125). CD47 levels are high on the surface of SCLC cells and preclinical data from human cell lines and xenografts suggest that blocking CD47 strongly promotes phagocytosis of SCLC cells by macrophages and inhibits tumor growth. Taken together these findings underlie the fact that the blockade of CD47 function may lead to tumor cell phagocytosis and elimination, validating CD47 as a potential target for cancer therapies (126).
Conclusions
SCLC often exhibits a dramatic response to platinum-based chemotherapy, only to later recur and become resistant to subsequent treatments. Despite these disappointing results, major progress has been made in the last decade in term of understanding of SCLC biology and advances in culturing SCLC tumors in vitro have greatly facilitated its study. The information derived from these biological studies represents the most promising avenue for new treatment strategies in SCLC. Even the issue of tumor tissue scarcity will be soon overcome with the use of circulating tumor cells (CTC) which are prevalent in SCLC and represent a readily accessible ‘liquid biopsy’. Coupling preclinical therapeutic studies using CTC-derived explants (CDX) with analysis of genetic progression is a potentially powerful approach to identify mediators of chemotherapy resistance and to target these pathways with rationally designed drugs. On the other hand, there are many issues related to the development and application of immunotherapy for SCLC, including high disease burden and lack of specific targets for vaccine-based treatments. Therefore, the optimal timing and schedule of immunotherapy in relation to other therapies (chemotherapy in particular) represent an important tool to improve disease control and enhance the development of an immune response against tumor.
Acknowledgements
Funding: R Rosell is supported by grants from the La Caixa Foundation and Red Tematica de Investigacion Cooperativa en Cancer (RTICC; grant RD12/0036/0072). S Pilotto and E Bria are supported by a Fellowship Award of the International Association for the Study of Lung Cancer (IASLC) and a grant of the Italian Association for Cancer Research (AIRC My First AIRC Grant No. 14282).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Berns A. Stem cells for lung cancer? Cell 2005;121:811-3. [PubMed]
- Pitt BR, Ortiz LA. Stem cells in lung biology. Am J Physiol Lung Cell Mol Physiol 2004;286:L621-3. [PubMed]
- Hong KU, Reynolds SD, Watkins S, et al. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol 2004;164:577-88. [PubMed]
- Reynolds SD, Giangreco A, Power JH, et al. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol 2000;156:269-78. [PubMed]
- Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005;121:823-35. [PubMed]
- Sutherland KD, Berns A. Cell of origin of lung cancer. Mol Oncol 2010;4:397-403. [PubMed]
- Pleasance ED, Stephens PJ, O'Meara S, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature 2010;463:184-90. [PubMed]
- Park KS, Liang MC, Raiser DM, et al. Characterization of the cell of origin for small cell lung cancer. Cell Cycle 2011;10:2806-15. [PubMed]
- Song H, Yao E, Lin C, et al. Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc Natl Acad Sci U S A 2012;109:17531-6. [PubMed]
- Sutherland KD, Proost N, Brouns I, et al. Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell 2011;19:754-64. [PubMed]
- Meuwissen R, Linn SC, Linnoila RI, et al. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell 2003;4:181-9. [PubMed]
- Borges M, Linnoila RI, van de Velde HJ, et al. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature 1997;386:852-5. [PubMed]
- Linnoila RI, Zhao B, DeMayo JL, et al. Constitutive achaete-scute homologue-1 promotes airway dysplasia and lung neuroendocrine tumors in transgenic mice. Cancer Res 2000;60:4005-9. [PubMed]
- Jiang T, Collins BJ, Jin N, et al. Achaete-scute complex homologue 1 regulates tumor-initiating capacity in human small cell lung cancer. Cancer Res 2009;69:845-54. [PubMed]
- Demelash A, Rudrabhatla P, Pant HC, et al. Achaete-scute homologue-1 (ASH1) stimulates migration of lung cancer cells through Cdk5/p35 pathway. Mol Biol Cell 2012;23:2856-66. [PubMed]
- Ball DW. Achaete-scute homolog-1 and Notch in lung neuroendocrine development and cancer. Cancer Lett 2004;204:159-69. [PubMed]
- Guillemot F, Lo LC, Johnson JE, et al. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell 1993;75:463-76. [PubMed]
- Li Y, Linnoila RI. Multidirectional differentiation of Achaete-Scute homologue-1-defined progenitors in lung development and injury repair. Am J Respir Cell Mol Biol 2012;47:768-75. [PubMed]
- Meder L, König K, Ozretić L, et al. NOTCH, ASCL1, p53 and RB alterations define an alternative pathway driving neuroendocrine and small cell lung carcinomas. Int J Cancer 2016;138:927-38. [PubMed]
- Augustyn A, Borromeo M, Wang T, et al. ASCL1 is a lineage oncogene providing therapeutic targets for high-grade neuroendocrine lung cancers. Proc Natl Acad Sci U S A 2014;111:14788-93. [PubMed]
- Osborne JK, Larsen JE, Gonzales JX, et al. NeuroD1 regulation of migration accompanies the differential sensitivity of neuroendocrine carcinomas to TrkB inhibition. Oncogenesis 2013;2:e63. [PubMed]
- Osborne JK, Larsen JE, Shields MD, et al. NeuroD1 regulates survival and migration of neuroendocrine lung carcinomas via signaling molecules TrkB and NCAM. Proc Natl Acad Sci U S A 2013;110:6524-9. [PubMed]
- Johnson BE, Makuch RW, Simmons AD, et al. myc family DNA amplification in small cell lung cancer patients' tumors and corresponding cell lines. Cancer Res 1988;48:5163-6. [PubMed]
- Nau MM, Brooks BJ, Battey J, et al. L-myc, a new myc-related gene amplified and expressed in human small cell lung cancer. Nature 1985;318:69-73. [PubMed]
- Polak P, Karlić R, Koren A, et al. Cell-of-origin chromatin organization shapes the mutational landscape of cancer. Nature 2015;518:360-4. [PubMed]
- Beasley MB, Lantuejoul S, Abbondanzo S, et al. The P16/cyclin D1/Rb pathway in neuroendocrine tumors of the lung. Hum Pathol 2003;34:136-42. [PubMed]
- McFadden DG, Papagiannakopoulos T, Taylor-Weiner A, et al. Genetic and clonal dissection of murine small cell lung carcinoma progression by genome sequencing. Cell 2014;156:1298-311. [PubMed]
- Dooley AL, Winslow MM, Chiang DY, et al. Nuclear factor I/B is an oncogene in small cell lung cancer. Genes Dev 2011;25:1470-5. [PubMed]
- Kwon MC, Berns A. Mouse models for lung cancer. Mol Oncol 2013;7:165-77. [PubMed]
- Yesner R. Heterogeneity of so-called neuroendocrine lung tumors. Exp Mol Pathol 2001;70:179-82. [PubMed]
- Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med 2010;134:1628-38. [PubMed]
- Debelenko LV, Brambilla E, Agarwal SK, et al. Identification of MEN1 gene mutations in sporadic carcinoid tumors of the lung. Hum Mol Genet 1997;6:2285-90. [PubMed]
- Fernandez-Cuesta L, Peifer M, Lu X, et al. Frequent mutations in chromatin-remodelling genes in pulmonary carcinoids. Nat Commun 2014;5:3518. [PubMed]
- Jones MH, Virtanen C, Honjoh D, et al. Two prognostically significant subtypes of high-grade lung neuroendocrine tumours independent of small-cell and large-cell neuroendocrine carcinomas identified by gene expression profiles. Lancet 2004;363:775-81. [PubMed]
- Le Treut J, Sault MC, Lena H, et al. Multicentre phase II study of cisplatin-etoposide chemotherapy for advanced large-cell neuroendocrine lung carcinoma: the GFPC 0302 study. Ann Oncol 2013;24:1548-52. [PubMed]
- Rossi G, Cavazza A, Marchioni A, et al. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol 2005;23:8774-85. [PubMed]
- Clinical Lung Cancer Genome Project (CLCGP); Network Genomic Medicine (NGM). A genomics-based classification of human lung tumors. Sci Transl Med 2013;5:209ra153.
- Travis WD, Brambilla E, Müller-Hermelink HK, et al., editors. Pathology & Genetics: Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC, 2004.
- Skov BG, Holm B, Erreboe A, et al. ERCC1 and Ki67 in small cell lung carcinoma and other neuroendocrine tumors of the lung: distribution and impact on survival. J Thorac Oncol 2010;5:453-9. [PubMed]
- Kreyberg L. Histological lung cancer types. A morphological and biological correlation. Acta Pathol Microbiol Scand Suppl 1962.Suppl 157:1-92. [PubMed]
- World Health Organization. Histological Typing of Lung Tumors, 2nd ed. Geneva: WHO, 1981.
- Nicholson SA, Beasley MB, Brambilla E, et al. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol 2002;26:1184-97. [PubMed]
- Travis WD. Advances in neuroendocrine lung tumors. Ann Oncol 2010;21 Suppl 7:vii65-71. [PubMed]
- Azzopardi JG. Oat-cell carcinoma of the bronchus. J Pathol Bacteriol 1959;78:513-9. [PubMed]
- Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol 2000;13:962-72. [PubMed]
- Travis WD. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod Pathol 2012;25 Suppl 1:S18-30. [PubMed]
- Hiroshima K, Iyoda A, Shida T, et al. Distinction of pulmonary large cell neuroendocrine carcinoma from small cell lung carcinoma: a morphological, immunohistochemical, and molecular analysis. Mod Pathol 2006;19:1358-68. [PubMed]
- Guinee DG Jr, Fishback NF, Koss MN, et al. The spectrum of immunohistochemical staining of small-cell lung carcinoma in specimens from transbronchial and open-lung biopsies. Am J Clin Pathol 1994;102:406-14. [PubMed]
- Agoff SN, Lamps LW, Philip AT, et al. Thyroid transcription factor-1 is expressed in extrapulmonary small cell carcinomas but not in other extrapulmonary neuroendocrine tumors. Mod Pathol 2000;13:238-42. [PubMed]
- Niederst MJ, Sequist LV, Poirier JT, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun 2015;6:6377. [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [PubMed]
- Peifer M, Fernández-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 2012;44:1104-10. [PubMed]
- Rudin CM, Durinck S, Stawiski EW, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet 2012;44:1111-6. [PubMed]
- George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47-53. [PubMed]
- Yokomizo A, Tindall DJ, Drabkin H, et al. PTEN/MMAC1 mutations identified in small cell, but not in non-small cell lung cancers. Oncogene 1998;17:475-9. [PubMed]
- Espinoza I, Miele L. Notch inhibitors for cancer treatment. Pharmacol Ther 2013;139:95-110. [PubMed]
- Kunnimalaiyaan M, Chen H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist 2007;12:535-42. [PubMed]
- Ito T, Udaka N, Yazawa T, et al. Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development 2000;127:3913-21. [PubMed]
- Sriuranpong V, Borges MW, Ravi RK, et al. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res 2001;61:3200-5. [PubMed]
- Sriuranpong V, Borges MW, Strock CL, et al. Notch signaling induces rapid degradation of achaete-scute homolog 1. Mol Cell Biol 2002;22:3129-39. [PubMed]
- Ntziachristos P, Lim JS, Sage J, et al. From fly wings to targeted cancer therapies: a centennial for notch signaling. Cancer Cell 2014;25:318-34. [PubMed]
- Chapman G, Sparrow DB, Kremmer E, et al. Notch inhibition by the ligand DELTA-LIKE 3 defines the mechanism of abnormal vertebral segmentation in spondylocostal dysostosis. Hum Mol Genet 2011;20:905-16. [PubMed]
- Geffers I, Serth K, Chapman G, et al. Divergent functions and distinct localization of the Notch ligands DLL1 and DLL3 in vivo. J Cell Biol 2007;178:465-76. [PubMed]
- Serth K, Schuster-Gossler K, Kremmer E, et al. O-fucosylation of DLL3 is required for its function during somitogenesis. PLoS One 2015;10:e0123776. [PubMed]
- Henke RM, Meredith DM, Borromeo MD, et al. Ascl1 and Neurog2 form novel complexes and regulate Delta-like3 (Dll3) expression in the neural tube. Dev Biol 2009;328:529-40. [PubMed]
- Saunders LR, Bankovich AJ, Anderson WC, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med 2015;7:302ra136.
- Pietanza MC, Spigel D, Bauer TM, et al. Safety, activity, and response durability assessment of single agent rovalpituzumab tesirine, a delta-like protein3 (DLL3)-targeted anibody drug conjugate (ADC), in small cell lung cancer (SCLC) [abstract LBA 7]. European Cancer Congress, Vienna, Austria, 2015.
- Hann CL, Rudin CM. Fast, hungry and unstable: finding the Achilles' heel of small-cell lung cancer. Trends Mol Med 2007;13:150-7. [PubMed]
- Poirier JT, Dobromilskaya I, Moriarty WF, et al. Selective tropism of Seneca Valley virus for variant subtype small cell lung cancer. J Natl Cancer Inst 2013;105:1059-65. [PubMed]
- Poirier JT, Gardner EE, Connis N, et al. DNA methylation in small cell lung cancer defines distinct disease subtypes and correlates with high expression of EZH2. Oncogene 2015;34:5869-78. [PubMed]
- Hubaux R, Thu KL, Coe BP, et al. EZH2 promotes E2F-driven SCLC tumorigenesis through modulation of apoptosis and cell-cycle regulation. J Thorac Oncol 2013;8:1102-6. [PubMed]
- Kim W, Bird GH, Neff T, et al. Targeted disruption of the EZH2-EED complex inhibits EZH2-dependent cancer. Nat Chem Biol 2013;9:643-50. [PubMed]
- Wee ZN, Li Z, Lee PL, et al. EZH2-mediated inactivation of IFN-γ-JAK-STAT1 signaling is an effective therapeutic target in MYC-driven prostate cancer. Cell Rep 2014;8:204-16. [PubMed]
- Byers LA, Wang J, Nilsson MB, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov 2012;2:798-811. [PubMed]
- Cardnell RJ, Feng Y, Diao L, et al. Proteomic markers of DNA repair and PI3K pathway activation predict response to the PARP inhibitor BMN 673 in small cell lung cancer. Clin Cancer Res 2013;19:6322-8. [PubMed]
- Mamdani H, Induru R, Jalal SI. Novel therapies in small cell lung cancer. Transl Lung Cancer Res 2015;4:533-44. [PubMed]
- Rosell R, Wannesson L. A genetic snapshot of small cell lung cancer. Cancer Discov 2012;2:769-71. [PubMed]
- Karachaliou N, Papadaki C, Lagoudaki E, et al. Predictive value of BRCA1, ERCC1, ATP7B, PKM2, TOPOI, TOPO-IIA, TOPOIIB and C-MYC genes in patients with small cell lung cancer (SCLC) who received first line therapy with cisplatin and etoposide. PLoS One 2013;8:e74611. [PubMed]
- Owonikoko TK, Zhang G, Deng X, et al. Poly (ADP) ribose polymerase enzyme inhibitor, veliparib, potentiates chemotherapy and radiation in vitro and in vivo in small cell lung cancer. Cancer Med 2014;3:1579-94. [PubMed]
- Owonikoko TK, Dahlberg SE, Khan SA, et al. A phase 1 safety study of veliparib combined with cisplatin and etoposide in extensive stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E2511). Lung Cancer 2015;89:66-70. [PubMed]
- Pietanza MC, Kadota K, Huberman K, et al. Phase II trial of temozolomide in patients with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin Cancer Res 2012;18:1138-45. [PubMed]
- Park KS, Martelotto LG, Peifer M, et al. A crucial requirement for Hedgehog signaling in small cell lung cancer. Nat Med 2011;17:1504-8. [PubMed]
- Behrens C, Lin HY, Lee JJ, et al. Immunohistochemical expression of basic fibroblast growth factor and fibroblast growth factor receptors 1 and 2 in the pathogenesis of lung cancer. Clin Cancer Res 2008;14:6014-22. [PubMed]
- Marek L, Ware KE, Fritzsche A, et al. Fibroblast growth factor (FGF) and FGF receptor-mediated autocrine signaling in non-small-cell lung cancer cells. Mol Pharmacol 2009;75:196-207. [PubMed]
- Schultheis AM, Bos M, Schmitz K, et al. Fibroblast growth factor receptor 1 (FGFR1) amplification is a potential therapeutic target in small-cell lung cancer. Mod Pathol 2014;27:214-21. [PubMed]
- Pardo OE, Wellbrock C, Khanzada UK, et al. FGF-2 protects small cell lung cancer cells from apoptosis through a complex involving PKCepsilon, B-Raf and S6K2. EMBO J 2006;25:3078-88. [PubMed]
- Zhang L, Yu H, Badzio A, et al. Fibroblast Growth Factor Receptor 1 and Related Ligands in Small-Cell Lung Cancer. J Thorac Oncol 2015;10:1083-90. [PubMed]
- Ishizaka Y, Itoh F, Tahira T, et al. Human ret proto-oncogene mapped to chromosome 10q11.2. Oncogene 1989;4:1519-21. [PubMed]
- Amoresano A, Incoronato M, Monti G, et al. Direct interactions among Ret, GDNF and GFRalpha1 molecules reveal new insights into the assembly of a functional three-protein complex. Cell Signal 2005;17:717-27. [PubMed]
- Rudin CM, Drilon A, Poirier JT. RET mutations in neuroendocrine tumors: including small-cell lung cancer. J Thorac Oncol 2014;9:1240-2. [PubMed]
- Sarvi S, Mackinnon AC, Avlonitis N, et al. CD133+ cancer stem-like cells in small cell lung cancer are highly tumorigenic and chemoresistant but sensitive to a novel neuropeptide antagonist. Cancer Res 2014;74:1554-65. [PubMed]
- Kolev VN, Xu Q, Padval M, et al. FAK and PI3K/mTOR Inhibitors Target Cancer Stem Cells: Implications for SCLC Treatment Strategies. Cancer Res 2015;75:1525.
- Umemura S, Mimaki S, Makinoshima H, et al. Therapeutic priority of the PI3K/AKT/mTOR pathway in small cell lung cancers as revealed by a comprehensive genomic analysis. J Thorac Oncol 2014;9:1324-31. [PubMed]
- Ben-Ezra JM, Kornstein MJ, Grimes MM, et al. Small cell carcinomas of the lung express the Bcl-2 protein. Am J Pathol 1994;145:1036-40. [PubMed]
- Ikegaki N, Katsumata M, Minna J, et al. Expression of bcl-2 in small cell lung carcinoma cells. Cancer Res 1994;54:6-8. [PubMed]
- Jiang SX, Sato Y, Kuwao S, et al. Expression of bcl-2 oncogene protein is prevalent in small cell lung carcinomas. J Pathol 1995;177:135-8. [PubMed]
- Gandhi L, Camidge DR, Ribeiro de Oliveira M, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol 2011;29:909-16. [PubMed]
- Rudin CM, Hann CL, Garon EB, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res 2012;18:3163-9. [PubMed]
- Faber AC, Farago AF, Costa C, et al. Assessment of ABT-263 activity across a cancer cell line collection leads to a potent combination therapy for small-cell lung cancer. Proc Natl Acad Sci U S A 2015;112:E1288-96. [PubMed]
- Christensen CL, Kwiatkowski N, Abraham BJ, et al. Targeting transcriptional addictions in small cell lung cancer with a covalent CDK7 inhibitor. Cancer Cell 2014;26:909-22. [PubMed]
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348:56-61. [PubMed]
- Antonia SJ, Mirza N, Fricke I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res 2006;12:878-87. [PubMed]
- Grant SC, Kris MG, Houghton AN, et al. Long survival of patients with small cell lung cancer after adjuvant treatment with the anti-idiotypic antibody BEC2 plus Bacillus Calmette-Guérin. Clin Cancer Res 1999;5:1319-23. [PubMed]
- Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med 2003;349:1543-54. [PubMed]
- Dalmau J, Graus F, Rosenblum MK, et al. Anti-Hu--associated paraneoplastic encephalomyelitis/sensory neuronopathy. A clinical study of 71 patients. Medicine (Baltimore) 1992;71:59-72. [PubMed]
- Roberts WK, Deluca IJ, Thomas A, et al. Patients with lung cancer and paraneoplastic Hu syndrome harbor HuD-specific type 2 CD8+ T cells. J Clin Invest 2009;119:2042-51. [PubMed]
- Koyama K, Kagamu H, Miura S, et al. Reciprocal CD4+ T-cell balance of effector CD62Llow CD4+ and CD62LhighCD25+ CD4+ regulatory T cells in small cell lung cancer reflects disease stage. Clin Cancer Res 2008;14:6770-9. [PubMed]
- Schultheis AM, Scheel AH, Ozretić L, et al. PD-L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer 2015;51:421-6. [PubMed]
- Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189-99. [PubMed]
- Hellmann MD, Creelan BC, Woo K, et al. 1229PD: Smoking history and response to nivolumab in patients with advanced NSCLCS. Ann Oncol 2014;25:iv429.
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [PubMed]
- Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 2013;24:75-83. [PubMed]
- Ishii H, Azuma K, Kawahara A, et al. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol 2015;10:426-30. [PubMed]
- Brezicka FT, Olling S, Nilsson O, et al. Immunohistological detection of fucosyl-GM1 ganglioside in human lung cancer and normal tissues with monoclonal antibodies. Cancer Res 1989;49:1300-5. [PubMed]
- Fuentes R, Allman R, Mason MD. Ganglioside expression in lung cancer cell lines. Lung Cancer 1997;18:21-33. [PubMed]
- McCaffery M, Yao TJ, Williams L, et al. Immunization of melanoma patients with BEC2 anti-idiotypic monoclonal antibody that mimics GD3 ganglioside: enhanced immunogenicity when combined with adjuvant. Clin Cancer Res 1996;2:679-86. [PubMed]
- Giaccone G, Debruyne C, Felip E, et al. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971-08971B; Silva Study). J Clin Oncol 2005;23:6854-64. [PubMed]
- Krug LM, Ragupathi G, Hood C, et al. Vaccination of patients with small-cell lung cancer with synthetic fucosyl GM-1 conjugated to keyhole limpet hemocyanin. Clin Cancer Res 2004;10:6094-100. [PubMed]
- Fischer JR, Schindel M, Bülzebruck H, et al. Decrease of interleukin-2 secretion is a new independent prognostic factor associated with poor survival in patients with small-cell lung cancer. Ann Oncol 1997;8:457-61. [PubMed]
- Levine BL. Performance-enhancing drugs: design and production of redirected chimeric antigen receptor (CAR) T cells. Cancer Gene Ther 2015;22:79-84. [PubMed]
- Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015;348:62-8. [PubMed]
- Jensen M, Berthold F. Targeting the neural cell adhesion molecule in cancer. Cancer Lett 2007;258:9-21. [PubMed]
- Jaiswal S, Jamieson CH, Pang WW, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009;138:271-85. [PubMed]
- Majeti R, Chao MP, Alizadeh AA, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009;138:286-99. [PubMed]
- Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 2012;109:6662-7. [PubMed]