Programmed cell death protein-1/programmed cell death ligand-1 pathway inhibition and predictive biomarkers: understanding transforming growth factor-beta role
Introduction
Non-small cell lung cancer (NSCLC) accounts for nearly 85% of all lung cancer cases and is commonly diagnosed at an advanced stage of disease. Even those patients undergoing potentially curative surgery can experience a recurrence, including systemic relapse, within few years, suggesting the systemic nature of the disease also in those patients with seemingly localized NSCLC (1,2). Different cytotoxic chemotherapy regimens are currently used to treat advanced NSCLC patients, but they only contribute with modest improvements in survival. During the last few years, the use of molecularly targeted agents, has dramatically improved the prognosis of lung cancer patients harboring specific oncogenic alterations, including EGFR mutations and ALK rearrangement. However, oncogene-directed therapies are currently used in the clinical setting only for relatively small subgroups of patients, mainly with adenocarcinoma histology. Furthermore, despite initial significant clinical benefit from EGFR- or ALK-tyrosine kinase inhibitors, patients will inevitably progress within 1−2 years, due to development of acquired resistance (3,4). Thus, additional treatment strategies that could obtain long lasting disease control without increasing toxicity are still needed. In recent years, further understanding of the interaction between the immune system and tumor growth has led to the development of several immunotherapies, with the goal to boost the host’s own immune anticancer response. These immunotherapies include immune checkpoint inhibitors, such as monoclonal antibodies directed against cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and programmed cell death protein-1 (PD-1)/programmed cell death ligand-1 (PD-L1) pathway, which have demonstrated therapeutic efficacy in a variety of human malignancies, including those historically considered as non-immunogenic, including lung cancer (5-7).
Immune response and cancer
Cancer cells harbor different genetic and epigenetic alterations; thus, a number of antigens that are potentially recognized and eliminated by the immune system are commonly expressed by tumors. Thymus-derived lymphocytes (T lymphocytes, T cells) activation and expansion are necessary for an effective adaptive immune response. Particularly, the main anti-tumor immune effector cells are represented by interferon-γ (IFN-γ)-secreting T cells, which are able to inhibit and kill malignant cells, thus impeding tumor growth and spread of the disease. Spontaneous lymphocytic infiltration is frequently observed in a variety of human cancers and in numerous studies tumor infiltrating lymphocytes (TILs) have been correlated with a more favorable clinical outcome of patients and also with response to treatment, including chemotherapy and immunotherapy (8-13). This can be explained by the fact that a component of this T-cell infiltrate is represented by tumor antigen-specific T cells activated in response to the growing tumors which exert their effector functions to eliminate cancer cells. However, in this model of T-cell infiltrated tumors, these cells subsequently become functionally inhibited by the effects of PD-L1 and indoleamine-2,3-dioxygenase (IDO) expression on tumor cells, driven by IFN-γ, and by the activity of T-regulatory (Treg) cells, thus contributing to immune escape (14). Immunologic responses are initiated when the antigens, presented by antigen presenting cells (APCs) in peptides complexed with major histocompatibility (MHC) complexes, are recognized by the T-cell receptor (TCR). Dendritic cells (DCs) are the most powerful APCs that migrate to lymph nodes after contact with tumor antigens and activate a tumor-specific-T-cell response (15). However, this first signal is not sufficient for activation of naïve T-cells. Additional co-stimulatory signals are required and are provided by the binding of CD28 on the T-cell surface with specific molecules, B7-1 (CD80) and B7-2 (CD86), on the APC (16). Once the T-cells are activated, the immune response enters the effector phase and T cells are capable of recognizing and destroying antigen-expressing tumor cells. The efficacy and duration of T-cell response depends on the balance between co-stimulatory and inhibitory signals that are delivered by different T-cell surface receptors. Immune co-stimulatory molecules include CD28, CD137, glucocorticoid-induced tumor necrosis factor (TNF) receptor (GITR), OX-40 and inducible costimulator (ICOS). Negative regulatory molecules or immune checkpoint molecules prevent overstimulation of immune responses and include cytotoxic T-lymphocyte antigen-4 (CTLA-4) and PD-1. These receptors interact with specific ligands of the B7 family: B7-1 (CD80) and B7-2 (CD86), that are present on APCs, but also on tumor cells. Immune checkpoints refer to molecules of inhibitory pathways that are crucial for maintaining self-tolerance and regulating the duration and amplitude of physiological immune responses against pathogens in periphery in order to avoid or minimize collateral tissue damage and inhibit chronic inflammation. CTLA4 and PD-1 represent the best characterized immune checkpoint receptors which deliver T-cell inhibitory signals (5,17). Inhibitory ligands are commonly overexpressed in APCs, tumor cells or other cells of the tumor microenvironment. Unfortunately, tumor cells can use these immune checkpoints as a defence mechanism. Indeed, it is well recognized that tumors, including lung cancers, are able to escape from immunosurveillance and maintain an immunosuppressive microenvironment through multiple mechanisms (18,19). These mechanisms include recruitment of regulatory cells (e.g., Treg cells, myeloid-derived suppression cells, and type 2 macrophages), production of molecules suppressing antitumor T-cell responses [e.g., interleukin-10 (IL-10), indoleamine 2,3-dioxygenase, transforming growth factor-β (TGF-β)], low antigen presentation and immunomodulation of T-cell response through down-regulation of co-stimulatory molecules or enhancement of co-inhibitory molecules (immune checkpoints) on T cells, other immune cells and tumor cells.
The role of immune checkpoint molecules
CTLA-4 is expressed exclusively on T-cells and shares identical ligands (CD80 and CD86) on APC with the T-cell co-stimulatory receptor CD28. When the TCR is engaged by a cognate antigen, CD28 binds CD80/CD86 and induces T-cell activation. However, CTLA-4 has a much higher overall affinity for both ligands and inhibits the activation of T-cells by competing with CD28 in binding CD80 and CD86 (5,20,21). CTLA-4 regulates the early stage of T-cell activation through additional mechanisms, such as recruitment and activation of the Src homology region 2 domain-containing phosphatase-2 (SHP2) and protein phosphatase 2A (PP2A) via the YVKM motif in its cytoplasmatic domain. This results in attenuation of kinase signaling, such as PI3K/AKT pathway, induced by TCR and CD28 (22-25). CTLA-4 primarily regulates CD4+ T cells by downregulating the activity of T helper cells and enhancing the immunosuppressive activity of Treg cells.
PD-1 is an immune checkpoint receptor expressed by activated T cells as well as B cells and natural killer (NK) cells. Similarly to CTLA4, PD-1 is highly expressed on Treg cells and may enhance their proliferation. Unlike CTLA-4, PD-1 primarily inhibits T-cell activity in the effector phase within peripheral tissues and tumors (5,26-30). Upon antigen recognition, activated T cells upregulate PD-1 expression on their surface. Specifically, PD-1 is expressed on a large proportion of TILs from many cancers. PD-1 signaling involves binding to specific ligands, including PD-L1 (or B7-H1), PD-L2 (or B7-DC) (31,32). PD-L1 is expressed on macrophages and can be induced by inflammatory cytokines, mainly IFN-γ on different cell types, including cancer cells, epithelial cells of various tissues, lymphoid cells and myeloid cells. Numerous tumor types express PD-L1, including NSCLC, suggesting that this pathway is activated in different cancers to contribute to anti-tumor immune evasion. Structurally, PD-1 has a cytoplasmatic Immunoreceptor Tyrosine-based Inhibitory Motif (ITIM) as well as an Immunoreceptor Tyrosine-based Switch Motif (ITSM) and has been found to be capable of recruiting the phosphatases SHP-1 and SHP-2 (33,34). Specifically, after binding to the ligand, PD-1 is able to recruit SHP-2 to the ITIM domain, resulting in inhibition of downstream TCR and CD28 signaling, mainly PI3K/AKT pathway activation. The effects of PD-1/PD-L1 interaction are inhibition of T-cell proliferation, survival and effector functions (cytokine release and cytotoxicity), induction of apoptosis of tumor-specific T-cell and promotion of differentiation of CD4+ T-cells into Tregs. Excessive induction of PD-1 on T cells in the setting of chronic antigen exposure can induce an exhausted or anergic state in T cells, as demonstrated in PD-1 expressing TILs.
Other immune checkpoint molecules with potential relevant roles in anti-tumor immune response have been characterized. These include T-cell Immunoglobulin and Mucin domain-containing protein 3 (TIM3; also known as HAVCR2), lymphocyte activation gene-3 (LAG-3) and V-domain immunoglobulin suppressor of T-cell activation (VISTA) (17).
Inhibition of the immune checkpoint pathways has been shown to reverse cancer immunosuppression and activate T cells, thus enhancing anticancer immune responses.
PD-1/PD-L1 pathway inhibitors in lung cancer
Different monoclonal antibodies targeting either PD-1 or PD-L1 are currently in advanced phases of clinical development in patients with different tumor types, including lung cancer.
Anti-PD-1 drugs
Nivolumab is a fully human IgG4 monoclonal antibody targeting PD-1. Nivolumab disrupts negative signaling triggered by PD-1 binding to PD-L1 or PD-L2 and restores T-cell antitumor function. PD-1 is expressed on T cells as well as on many other immunologic cells, including B cells and NK cells, and therapeutic blockade of the PD-1 pathway may also influence the function of these cells (6). In a phase I nivolumab study, response rates were 18% in NSCLC, 28% in melanoma, and 27% in renal cell cancer. The drug was well-tolerated, with grade 3 or 4 adverse events observed in 14% of patients (35). Expansion cohorts of patients with heavily pretreated NSCLC demonstrated a response rate of 17%, with median duration of response of 17 months. Responses were rapid, with 50% of responders detected at the first response evaluation (8 weeks after starting treatment). Response rates were similar in squamous and non-squamous NSCLC. Median progression-free survival (PFS) for responders was 20.6 months. Median overall survival (OS) was 9.9 months. Survival rates at 1-, 2- and 3-year were 42%, 24% and 18% respectively (36). Common side effects were fatigue, anorexia, and diarrhea (similar in frequency to the overall study population); grade 3−4 toxicity was found in 14% and pneumonitis in 7% of NSCLC patients (3% had grade 3/4 pneumonitis). Three treatment-related deaths occurred among patients with NSCLC, each associated with pneumonitis.
In a phase II trial enrolling patients with advanced squamous NSCLC who had received two or more prior treatments, nivolumab was associated with a 14.5% response rate after an 11-month follow-up, with 3.3-month median time to onset of response (37). Responses were durable, with 77% of responders presenting ongoing responses during the analysis. Some of these patients (59%) had durable responses of 6 months or longer. Phase III trials in NSCLC have now been completed and results have been reported. The phase III CheckMate 017 trial was stopped early in January 2015 following an assessment conducted by the independent Data Monitoring Committee demonstrating a superior 3-month OS of nivolumab compared with docetaxel in patients with advanced squamous NSCLC pretreated with platinum-based chemotherapy (38). The median OS was 9.2 months with nivolumab vs. 6.0 months with docetaxel [hazard ratio (HR), 0.59; 95% CI, 0.44 to 0.79; P<0.001]. The response rate was 20% with nivolumab vs. 9% with docetaxel (P=0.008) and the median PFS was 3.5 months with nivolumab vs. 2.8 months with docetaxel (HR, 0.62; 95% CI, 0.47 to 0.81; P<0.001). The toxicity profile of nivolumab was more favourable than docetaxel. Benefit was independent of PD-1 expression. Recently, according to the results of the CheckMate 057 trial, nivolumab has been demonstrated to be the first PD-1 inhibitor to significantly improve OS in comparison with docetaxel (12.2 vs. 9.4 months; HR 0.73; 95% CI, 0.59 to 0.89; P=0.002), in previously treated patients with advanced non-squamous NSCLC with 27% reduction in risk of death and significantly improved overall response rate. In this study, tumor PD-L1 expression was found to be predictive of nivolumab benefit (39). Other studies are ongoing, including the phase III trial comparing nivolumab to chemotherapy in first-line PD-L1-positive metastatic NSCLC (CheckMate 026, NCT02041533) and a phase I multi-arm trial testing the safety and efficacy of nivolumab in NSCLC in combination with ipilimumab or standard chemotherapy or erlotinib or bevacizumab (CheckMate 012, NCT01454102).
Pembrolizumab (MK-3475), is a monoclonal anti-PD-1 humanized IgG4 antibody approved for the treatment of metastatic melanoma. Results from a phase I study in previously treated and untreated patients with advanced NSCLC showed that pembrolizumab was generally well-tolerated and provided robust antitumor activity. Overall response rate was 19.4% by RECIST 1.1 and the median duration of response was 12.5 months. Median PFS and OS were 3.7 and 12 months respectively. A proportion score of at least 50% of PD-L1 expression was associated with a higher response rate and longer PFS and OS than was a proportion score of less than 50%, which indicates that this is a subgroup of patients in whom the PD-L1 pathway can be successfully targeted. The toxicity profile was acceptable, with 9.5% of patients experiencing ≥ grade 3 adverse events. Pneumonitis of grade 3 or greater was observed in nine patients (1.8%), including one (0.2%) who died (40). Other trials are ongoing, including a phase II/III trial comparing 2 dose levels of pembrolizumab with docetaxel in PD-L1 positive NSCLC patients who have received ≥ one prior treatment regimen (NCT01905657) and other studies that compare pembrolizumab to platinum-based chemotherapy combinations as first-line treatment for PD-L1 positive NSCLC patients (NCT02142738 and NCT02220894).
Anti-PD-L1 drugs
Targeting PD-L1 with specific monoclonal antibodies has also shown to be a very promising approach similar to targeting PD-1. However, PD-L1 inhibition may result in different biological effects than those obtained from targeting PD-1: first, PD-L1 antibodies do not prevent PD-1 from interacting with PD-L2, although the effect of this interaction remains to be determined; second, PD-L1 blockade could also prevent the interaction of PD-L1 with the B7-1, thus suppressing an additional negative control on T cells (6,41).
BMS-936559 was the first anti-PD-L1 antibody demonstrating activity in a variety of advanced solid tumors. In a phase I trial, BMS-936559 treatment of NSCLC was associated with an objective response rate (ORR) of 10% (5 of 49 NSCLC patients evaluable: four patients with the non-squamous and one with the squamous subtype). Three of these patients had responses lasting at least 24 weeks. Stabilization of disease at 6 months was observed in 12% of patients. In the overall patient population, grade 3 or 4 toxic effects related to treatment occurred in 9% of patients (42).
MEDI4736 is an engineered human IgG1 monoclonal antibody that blocks PD-L1 from binding to PD-1, thus allowing T cells to recognize and kill malignant cells. Interim results from the NSCLC cohort of an ongoing phase I, first-in-human study in patients with advanced solid tumors (NCT01693562), showed preliminary clinical activity of MEDI473 (43). At data cut-off, 155 NSCLC patients were treated with the drug in the dose escalation and expansion cohorts, of whom 143 received 10 mg/kg every 2 weeks. The majority of patients had received one prior systemic treatment and had non-squamous NSCLC. Early and durable activity was observed in both squamous and non-squamous NSCLC. Response rate by Response Evaluation Criteria in Solid Tumors (RECIST) was 13% and preliminary data indicated that PD-L1 expression may correlate with response and disease control rate. The drug was well tolerated, with no adverse events leading to discontinuation and low rates (4%) of grade 3/4 adverse events. No drug-related colitis and no grade 3/4 pulmonary toxicities were observed (43).
Ongoing trials are evaluating MEDI4736 in different settings of disease, including a phase III trial of the drug following concurrent chemoradiotherapy for unresectable stage III NSCLC (NCT02125461) and a phase III trial for pretreated, advanced NSCLC patients to test the combination of MEDI4736 with tremelimumab for PD-L1-negative patients and MEDI4736 as a single agent for PD-L1-positive patients vs. chemotherapy (NCT02352948).
Atezolizumab (MPDL3280A) is another engineered IgG anti-PD-L1 antibody, with modified Fc domain that prevents antibody-dependent cell-mediated cytotoxicity, that has shown activity in NSCLC. In a phase I trial, an ORR of 22% was observed in patients with previously treated metastatic NSCLC, with both squamous and non-squamous histology, including several patients exhibiting rapid tumor shrinkage (44). All responses were ongoing or improving at the time of analysis and the 24-week PFS was 46%. The safety profile was tolerable with no grade 3/4 pneumonities. In an updated analysis of the phase I trial, 85 patients with NSCLC were included in the safety analysis and 53 in the efficacy analysis. The ORR in the NSCLC cohort was 23% and all responses were maintained for the duration of treatment (median 48 weeks). Interestingly, the authors reported a higher ORR of 26% for patients who had smoked as compared with patients who had never smoked (ORR =10%). This could be explained by evidence demonstrating that, in comparison with non-smokers, smokers might bear tumors with a high mutation rate, significantly increasing their immunogenicity (45).
Biomarkers of response to PD-1/PD-L1 inhibitors
Identification of predictive biomarkers to select patients for immune therapies is currently being investigated; however it is difficult to establish the role of a single biomarker due to the complexity of the immune response which involves a great amount of interrelated molecules and immune cells, each of which dealing with a different crucial activity. Because of the role of PD-1/PD-L1 pathway in downregulating the activity of effector T cells within tumors and peripheral tissues, most biomarker investigations have focused on tumor microenvironment components. Tumor microenvironment is defined as being characterized by the crosstalk between different cell types, including tumor cells, inflammatory cells, T cells, B cells, NK cells, myeloid cell populations, tumor-associated fibroblasts (TAFs) and stromal cells. The most studied biomarker has been PD-L1 expression on tumor cells, due to its crucial role in immune response modulation by inhibiting the activity of tumor infiltrating cytotoxic T cells, thus creating a local immunosuppressive milieu.
Several studies have demonstrated that patients whose tumors express PD-L1, as detected by immunohistochemistry, have higher response rates to PD-1/PD-L1 blockade therapy than patients who do not express PD-L1 (35,40,43,45,46). As commented above, activated tumor antigen-specific T cells produce interferons which induce PD-L1 expression on tumor cells. The presence of pre-existing PD-1 positive T cells with tumor antigen specificity which become inactivated upon binding with PD-L1 is crucial for responses to PD-1 blockade therapy (acquired immune resistance) (5). This mechanism differs from others in which tumor PD-L1 expression is not correlated to the presence of effector cells and to PD-1 expression on these cells. Indeed, tumor cells can activate PD-L1 expression independently of inflammatory signals via multiple oncogenic signaling pathways, including PI3K/AKT, ALK/STAT3, MEK/ERK/STAT1 (defined as innate immune resistance) (47-49).
However, in many of these trials, albeit at a lower rate, responses have also been observed in patients with PD-L1-negative tumors, suggesting that other factors could explain immunotherapy activity in this subgroup of patients. In a seminal study exploring the predictive value of multiple immune biomarkers in pretreatment samples from nivolumab-treated patients with different advanced solid tumors, PD-L1 expression on tumor cells emerged as the strongest predictive factor associated with response to the antibody (46). The presence of TILs was not demonstrated to be predictive of anti-PD-1 response, in contrast to others demonstrating a correlation of the numbers of TILs with ipilimumab activity in melanoma (13). In a recent study, Herbst et al. observed that the association of PD-L1 expression by infiltrating immune cells with objective response to the anti-PD-L1 monoclonal antibody MPDL3280A was stronger than that with tumor PD-L1 expression, suggesting that a pre-existing T-cell activity suppression could be crucial in mediating response to the anti-PD-L1 monoclonal antibody (50). However, results in terms of PD-L1 expression predictivity to immunotherapies are difficult to interpret, because of the lack of a standardized immunohistochemistry assay, different cut-off levels to determine PD-L1 positivity, variability in the timing of biopsy collection, in sample processing and preservation across all the reported studies. In addition, tumors are heterogeneous and the sample used for the assay may not be representative of the whole tumor. Other potential predictive biomarkers of response to immunotherapies have been identified, including the presence of CD8+ T-cell infiltration and the expression of PD-L1 and PD-1 in immune cells at the invasive tumor margin that correlated with response to pembrolizumab in melanoma (51). Recent evidence suggests that response to immunotherapies may rely on a tumor specific genomic landscape. Indeed, every tumor contains a variable number of somatic mutations and some tumors, including melanomas and lung cancers, are characterized by very high numbers of somatic mutations associated with environmental exposure to ultraviolet light and smoking. Somatic mutations can generate neoantigens within tumors that can trigger specific immune responses. A higher mutational load as assessed by whole-exome sequencing in NSCLC samples from patients was strongly associated with response to pembrolizumab. Efficacy was also correlated with a molecular smoking marker, specific DNA repair pathway mutations, and a higher burden of candidate neoantigens (52). Epithelial-mesenchymal transition (EMT) has been correlated with cancer progression, metastasization and drug resistance. In a recent work, it was demonstrated that EMT is correlated with CD8+ TIL immunosuppression and metastasis through a mechanism involving ZEB1 and miR-200 loss that controls PD-L1 expression in lung cancer cells (53).
TGF-β signalling pathway
The TGF-β superfamily are cytokines that bind to a heterodimeric receptor complex consisting of type I (TβRI, activin-line receptor kinase family) and type II (TβRII) transmembrane serine/threonine kinase receptors. TGF-βs are secreted by tumor cells, immune cells and other nonhematopoietic cells; three isoforms of TGF-β have been identified of which TGF-β1 is predominantly expressed in the immune system (54). The TGF-β ligands and receptors are widely expressed in different tissues and tumors, playing an important role in the maintenance of immune homeostasis and the coordination of responses to injury and stress. Unlike most cytokines, TGF-β is synthesized as an inactive precursor, which forms a homodimer that interacts with its latency-associated peptide (LAP) and a latent TGF-β-binding protein (LTBP), forming a larger complex called the large latent complex (LLC). The TGF-β activation process involves the release of the LLC from the extracellular matrix (ECM), followed by further proteolysis of LAP to release active TGFβ to its receptors. Matrix metalloproteinase 2 (MMP2), MMP9 and thrombospondin 1 (THBS1) are known to cleave latent TGF-β. Alternatively, interaction with integrins or pH changes in the local environment are known to activate latent TGF-β and free active TGF-β (55). Binding of TGF-β with the heterodimeric receptor complex activates intracellular signaling involved in the control of crucial mechanisms, including proliferation, differentiation, migration, invasion, EMT, ECM remodelling and immune-suppression (56). Intracellular signal transduction is mediated by phosphorylation of specific transcription factors known as Smads (57). Phosphorylated Smad2 and Smad3 combine with Smad4 to modulate gene transcription by entering the nucleus, binding to the target genes promoters and recruiting histone acetyl transferase or histone deacetylase. Smad7 is a negative regulator of the Smad signalling pathway, that binds to ubiquitin specific peptidase 15 (USP 15) as a complex with SMAD specific E3 ubiquitin protein ligase (SMURF2), leading to enhanced TGF-β signalling. Other Smad-independent signalling molecules and pathways can be regulated by TGF-β, including Ras-Mek-Erk, Rho GTPase, PI3K/AKT, TGF-β-activated kinase 1 (TAK1), p38, JUN N-terminal kinase (JNK) and nuclear factor-κB. (NF-κB) (58). TGF-β is a pleiotropic cytokine that exerts its function on several cells types; therefore, dysfunction of TGF-β signalling could play a role in multiple biological processes and pathologies, including carcinogenesis, fibrosis, wound healing and immune responses (54,55,59-61).
The role of TGF-β in immune response
TGF-β has a pivotal role within the immune system; it is mainly involved in suppressing the immune response in the periphery, thus maintaining immune self-tolerance and preventing autoimmune disease. Therefore it has been correlated with host immunosurveillance inhibition. As a pleiotropic cytokine, TGF-β maintains immune homeostasis through regulation on essentially every cell type of the innate and adaptive immune system (Figure 1). Specifically, TGF-β suppresses the proliferation, differentiation and effector functions of multiple immune cell types, especially T lymphocytes, and induces the generation of immunosuppressive cells or phenotypes (55,62). Experiments in transgenic mice have demonstrated the critical role for TGF-β in regulating suppression of conventional CD4+ and CD8+ T cells. A direct correlation between the frequency of cytotoxic T lymphocytes (CTLs) in TILs, mainly activated cytotoxic CD8+, and the OS has been demonstrated in patients with different types of cancer (9-12,63). TGF-β signalling dampens tumor-specific CTLs function and frequency in tumors and blocking TGF-β signalling on CD4+ and CD8+ T cells is associated with a strong antitumor response due to enhanced proliferation and activity of tumor-specific CTLs (62,64).
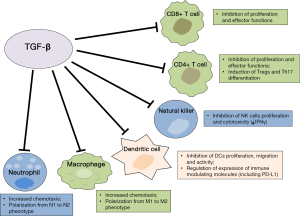
TGF-β has been demonstrated to exert its activity by regulating T cell proliferation, differentiation and survival through multiple mechanisms. TGF-β has been shown to suppress T-cell proliferation through Smad3 transcription factor-dependent blockade of the production of IL-2, which is a known cytokine able to activate T cells, NK, and other types of cells of the immune system (54,65). However, other IL-2-independent mechanisms involved in T-cell proliferation inhibition have been described, including Smad3 binding to myc-promoter and decreased expression of other cell-cycle promoting factors and up-regulation of cyclin-dependent kinase inhibitors, including p15 and p21 (54,66). TGF-β markedly suppresses the “cytotoxic program” of CTLs through transcriptional repression of genes encoding key proteins involved in the effector functions, including perforin, granzymes, IFN-γ and other cytotoxins (62,67). Recently, one of the mechanisms involved in T cell unresponsiveness driven by TGF-β has been described in cancer cell lines and mice models (68). In this study, upregulation of the forkhead box protein 1 (Foxp1) was demonstrated in human and mouse tumor-infiltrating effector CD8+ T cells. Foxp1 inhibited T-cell proliferation and impaired T-cell effector functions by decreasing cytolytic Granzyme-B and IFN-γ production in response to antigens. These effects were mediated by interaction of Foxp1 with Smad2 and Smad3, induced in response to microenvironmental TGF-β. These results suggest Foxp1 is required for the suppressive activity of TGF-β on CD8+ T cells, including transcriptional repression of c-myc and c-jun (68).
TGF-β also contributes to immunosuppression because of its significant impact on CD4+ T-cell differentiation and functions. Indeed, TGF-β, as well as other suppressing cytokines present at high concentrations in tumor microenvironment, have a crucial role in inducing Treg cells generation (55,62). Tregs are diverse populations of lymphocytes that physiologically maintain peripheral tolerance and immune homeostasis. These cells are found at higher frequencies in peripheral blood, lymph nodes and tumor sites of cancer patients and can suppress antitumor immune response (54,62,69). Tregs are divided mainly in two distinct subsets: natural Treg (nTreg) cells and adaptive or induced Treg (iTreg). nTreg are thymus-derived T cells that arise during early stages of human fetal development and maintain self-tolerance in an antigen-independent manner. iTreg cells, which include a great variety of cells stimulated by different cytokines, are induced in the periphery (iTreg) from naïve T cells in response to self- or tumor-antigens stimulation (70,71). Tregs are CD4+ cells characterized by the expression of CD25, the alpha chain of IL-2 receptor. The expression of trascription factor Foxp3 has been demonstrated to be necessary for the development and proper function of these cells (71,72). nTreg also express other markers, including adhesion molecules, CTLA-4, PD-1, chemokine receptors (CCR7, CXCR4), CD28 and GITR-related protein (5,71,73). These cells are able to suppress proliferation and cytokine secretion of conventional CD4+ effector T cells and they can also suppress other immune cell types, including CD8+ T cells, NK cells, monocytes/macrophages, B cells (71,74).
Different studies in vitro have demonstrated that TGF-β induces up-regulation of Foxp3 expression in T cells stimulated via TCR, thus generating cells with suppressive properties (iTreg). IL-2 is necessary for the conversion of naïve T cells to Foxp3+ T cells (75,76). NF of activated T cells (NFAT) and Smad3 are crucial for Foxp3 induction in iTregs, however, Foxp3 expression can also be positively regulated by relief from GATA-3 mediated transcriptional inhibition of its promoter by competition with the TGF-β-induced Id3 (77,78). TGF-β could be involved in generating Tregs in vivo and may assist these cells in suppressing CTL effector function in the tumor microenvironment, as demonstrated in mouse models (54,62). High levels of Tregs within tumors have been correlated with poor prognosis in several tumor types, including lung cancer (79-81), but the role of TGF-β in Tregs generation in cancer patients remains to be determined. Since several tumors can produce TGF-β, the induction of Treg cells by this cytokine might be a mechanism by which tumors escape the antitumor immune response, thus suggesting new potential anti-tumor immunotherapeutic strategies. However, by using microarray techniques, it was demonstrated that the signature of genes expressed by TGF-β-induced-Foxp3+ T (iTreg) cells differed from that of nTreg, suggesting the need for further studies before assessing their potential role for therapeutic approaches to human diseases (75).
In addition, TGF-β inhibits Th1 and Th2 cell differentiation which are crucial in increasing the CTL-mediated antitumor response and, together with IL-6, promotes Th17 cell differentiation (55,82,83). It is presumed that IL-6 inhibits Tregs development with stimulation of Th17. TGF-β, coordinating with IL-21, induces CD4+, CD25+ Tregs that counterbalance the effect of IL-6. Th17 is a newly defined Th-cell population that expresses IL-17 and regulates leukocyte recruitment and activation. However, the functions of Th17 cell types in tumor biology remains controversial and also the role of TGF-β in Th17 differentiation has been debated in more recent studies (55,84). TGF-β has been demonstrated to also affect B-cell, by suppressing their proliferation and modulating their activation and secretion of Immunoglobulins (62).
DCs are the most powerful antigen-presenting cells (APCs) and they are able to initiate antigen-specific immune responses by presenting antigens to T cells and by activating B and NK cells. DCs are at the center of the immune system owing to their ability to control both tolerance and immunity (85). Upon stimulation, DCs up-regulate MHC II, costimulatory receptors and cytokines, including IL-12, and interact with T-cells in order to induce their activation and differentiation. TGF-β affects DC biology in several ways (62). TGF-β can reduce DCs motility, thereby interfering with the migration and antigen transportation to draining lymph nodes for presentation to adaptive immune cells and it can also directly induce DC apoptosis. DCs secrete TGF-β and respond to TGF-β produced by tumor cells, either in autocrine or paracrine manner, by down-regulating expression of MHC II and co-stimulatory molecules and cytokines, including CD40, CD80 and CD86, tumor-necrosis factor, IFNα, IL-12 and CC-chemokine ligand 5 (CCL5) (62,86). These tolerogenic DCs are able to promote the formation of Tregs and this capacity is increased by TGF-β (62,87-89). These effects may result in inhibition of T-cell activation and contribute to the generation of a local immune suppressive milieu and tumor escape from immune system.
In in vitro experiments exploring the role of TGF-β in lung tumor microenvironment, TGF-β also upregulated the expression of immune modulation molecules, including PD-L1 and GITR-related protein ligand, on DCs (90). In a recent work, TGF-β was shown to upregulate PD-L1 expression on DCs via activation of STAT3 phosphorylation and signaling activation (91). Several studies have linked PD-L1 expression on DCs with the induction of immune tolerance and tumor evasion from immune response. The binding of PD-L1 with PD-1 expressed on T cells is responsible for T-cell immune activity suppression. PD-L1 expression has been demonstrated to be linked to the development and activation of Tregs. In this study, TGF-β-DCs were able to induce CD4+, CD25+, Foxp3+ Treg generation and apoptosis of T cells, thus diminishing the ability of DCs to activate a tumor-antigenic specific CTL response. A positive correlation between Treg percentage and PD-L1 expression was found (91).
With respect to innate immunity system cells, TGF-β inhibits NK-cell proliferation and function. TGF-β directly suppresses IFNγ production by NK cells through transcriptional effects of SMAD3 on the IFNγ promoter (92). Furthermore, TGF-β inhibits NKp30 and NKG2D receptor expression, resulting in decreased NK-cell cytolytic activity (93,94).
TGF-β also has a role in regulating myeloid lineage cells within tumors. Solid tumors are composed of approximately 50% macrophages and high levels of tumor-associated macrophages (TAMs) are correlated with poor cancer prognosis. TAMs are a heterogeneous population and are typically divided in different phenotypes with different activities: M1 are activated by IFN-γ and lipopoly saccharide (LPS) and are efficient at presenting antigens and have immunostimulating activity by secretion of active cytokines, including IL-12; in contrast, M2 include a variety of macrophages exerting suppressive function, that are mainly involved in wound healing and promote angiogenesis and tumor progression, similar to myeloid-derived suppressor cells; M2 are mainly induced by IL-10, IL-4 and IL-13 (95-97). TAMs acquire their phenotype by expressing high levels of TGF-β, IL-10, CXC-chemokine ligand 10 (CXCL10) and CXCL9 and other IFN-γ-responsive genes. In skin cancer, TGF-β recruits TAMs and this contributes to immune escape and tumor progression (98). TGF-β promotes M1 to M2 phenotype differentiation and this can be associated to down-regulation of NF-κB expression and activation (62). TGF-β also affects neutrophils migration and cytotoxicity (99,100). Neutrophils have important functions during inflammation and, although their role in tumor progression needs to be determined, those infiltrating tumor associated neutrophils (TANs) are polarized into two different phenotypes (N1 and N2), similar to TAMs. N2 cells promote tumor angiogenesis and metastasis and produce reactive oxygen species (ROS) and pro-inflammatory cytokines that inhibit the antitumor immune response by leading to oxidative damage and inhibition of T-cell function. TGF-β has been demonstrated to influence polarization of neutrophils within tumor microenvironment (62,101).
Gr-1+, CD11b+ immature myeloid cells are also called myeloid derived suppressor cells (MDSCs) and play an important suppressive effect through inhibition of T-cell activation but also NK, DCs and B cells, through the production of arginase and ROS. Tumors utilize numerous pathways to inhibit immune responses, including the elaboration of immune-suppressive mediators such as prostaglandin E2 (PGE2), TGF-β, IL-10, vascular endothelial growth factor (VEGF), granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-6 and stem cell factor (SCF), which recruit and/or activate MDSCs. Solid tumors contain MDSCs that maintain an immune-suppressive network in the tumor microenvironment and compromise the efficacy of cancer immunotherapy (102,103). MDSCs could also affect tumor progression; in fact, they produce high levels of MMPs and TGF-β and contribute to tumor growth, neovascularization and metastasization (104,105).
The role of TGF-β in tumor progression and EMT
High levels of TGF-β are produced by many hematologic and solid tumors, including breast, colon, liver and lung and TGF-β overexpression has been correlated with metastasis, poor prognosis and resistance to therapy. In addition, mutations of TGF-βRs as well as Smad proteins have been found in several cancer types (54,59,106). During tumorigenesis, TGF-β acts both as a tumor suppressor in earlier stages and as a tumor promoter during later stages (54,107-109); the mechanisms underlying this dual role remain to be elucidated. In early stages, TGF-β can suppress tumorigenesis by inhibiting cell cycle progression and stimulating apoptosis. In contrast, it has been reported that later stages of cancer progression are characterized by increased expression or activation of the TGF-β pathway by tumor cells in which it contributes to modulate cancer-inducing processes. One of the hallmarks of cancer is that the vast majority of cases exhibits insensitivity to TGF-β-mediated growth inhibition. TGF-β is involved in EMT induction, angiogenesis and metastasization. As mentioned above, EMT enhances cellular migration and invasive properties, as cell migration requires loss of cell-cell contacts and acquisition of fibroblastic characteristics. EMT can also be associated with the acquisition of cancer “stem cell-like” properties and, therefore, more aggressive tumor cells and worse response to treatments, including radiotherapy and chemotherapy (110,111). Recently, NSCLC samples with EMT features, as determined by a specific gene expression signature, were found to be strongly associated with upregulation of multiple immune-activating and immune-modulatory molecules, including IL-6, IL-10, CTLA-4, IDO, compared to epithelial NSCLC samples. In addition, EMT was associated with higher expression of PD-L1 on tumor cells, suggesting that immunotherapies targeting immune checkpoints might be beneficial for these subgroups of NSCLC patients with mesenchymal characteristics (112).
The anti-tumor immunity suppression by TGF-β may contribute importantly to cancer progression and resistance to existing therapies. In experiments in melanoma cell lines and murine model, a correlation between hypoxia-induced upregulation of the stemness-associated transcription factor Nanog, TGF-β and immunosuppression has been demonstrated (113). Hypoxia is commonly observed in tumor microenvironment and it is responsible of acquisition of stem-cell like properties by tumor cells and promotion of tumor tolerance through regulation of multiple immune cells. Nanog was involved in the control of tumor growth following treatment with specific therapeutic vaccination (114). The same group demonstrated that hypoxia-induced Nanog correlated with acquisition of stem cell-like properties in tumor cells. Furthermore, under hypoxic conditions, it enhanced TGF-β expression by binding with its promoter and, through TGF-β, promoted the differentiation of CD4+ T naïve cells into Tregs and the macrophages immunosuppressive phenotype. Nanog targeting significantly reduced Tregs and macrophages and increased CD8+ T effector cells infiltrating in tumor bed, thus suggesting new potential strategies to enhance immune therapies.
Conclusions
Immune checkpoint inhibition is a promising strategy in lung cancer, inducing durable responses with manageable toxicities. However, the modest ORR suggests the need to further improve their therapeutic efficacy. To this aim, it is of paramount importance to identify predictive biomarkers to select target patients that may most benefit from checkpoint blockade. Immune response is regulated by many molecules and immune cells within the tumor microenvironment, which can also have a role in modulating response to anti-PD-1 and anti-PD-L1 monoclonal antibodies. PD-L1 expression in tumor cells has emerged as the most promising candidate for predicting response to these drugs, however, the lack of PD-L1 expression can not be relied upon to exclude patients from these highly effective immunotherapeutic approaches. The role of other components of tumor microenvironment in modulating the response to anti-PD-1 and anti-PD-L1 antibodies is being deeply investigated. TGF-β is a tolerogenic cytokine that is frequently overexpressed in aggressive cancers and plays a crucial role in the maintenance of immune homeostasis and in tumorigenesis. Its pivotal function is to suppress the immune response in periphery through its multiple effects on several cell types. TGF-β has suppressive effects on Th1 and cytotoxic T-cells, while promoting the generation and activity of Treg cells. In innate immunity, TGF-βs increase the suppressive activity of monocytes/macrophages by promoting polarization towards an M2 phenotype and decrease the cytotoxicity of NK cells. In addition, TGF-β affects antigen presentation by DCs by modulating the expression of MHC II and also induces upregulation of immune modulating molecules, including PD-L1, with the induction of immune tolerance and tumor evasion from immune response. Furthermore, TGF-β has been correlated with EMT phenotype that can play a crucial role in cancer progression and resistance to treatments. Recently, lung cancers with EMT phenotype have been demonstrated to have higher expression of PD-L1 and other markers of inflammatory response. A close relation exists between the immunologic system, hypoxia, acquisition of stemness properties, and EMT induced by TGF-β, thus suggesting new opportunities for enhancing immune therapies. Although some evidence suggests a potential role for TGF-β in modulating response to specific anti-PD-1/PD-L1 monoclonal antibodies, due to the pleiotropic activity of TGF-β, its role in immunoresistance is highly complex and context-dependent and still needs to be explored. Indeed, preclinical studies have shown that TGF-β antagonists can significantly suppress tumorigenesis and/or metastasis by effective antitumor immunity reactivation mechanisms. For the same reasons, TGF-β antagonism can be able to improve the efficacy of immunomodulatory chemotherapy and of immunotherapeutic agents. Understanding the intricate signalling pathways and multiple biological processes controlled by TGF-β could provide new insights about the role of microenvironment during immune response. However, the role of TGF-β inhibitors, also in association with vaccines or anti-PD-1/PD-L1 or CTLA-4 specific antibodies and the optimal timing of administration of these immunotherapies in the clinical setting still need to be determined.
Beyond TGF-β, deeper understanding of the immune features of lung cancers will contribute to the identification of more reliable predictive markers for assessing response to checkpoint inhibitor therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rena O, Oliaro A, Cavallo A, et al. Stage I non-small cell lung carcinoma: really an early stage? Eur J Cardiothorac Surg 2002;21:514-9. [PubMed]
- Santarpia M, Altavilla G, Pitini V, et al. Personalized treatment of early-stage non-small-cell lung cancer: the challenging role of EGFR inhibitors. Future Oncol 2015;11:1259-74. [PubMed]
- Rosell R, Bivona TG, Karachaliou N. Genetics and biomarkers in personalisation of lung cancer treatment. Lancet 2013;382:720-31. [PubMed]
- Santarpia M, Gil N, Rosell R. Strategies to overcome resistance to tyrosine kinase inhibitors in non-small-cell lung cancer. Expert Rev Clin Pharmacol 2015;8:461-77. [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [PubMed]
- Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol 2015;33:1974-82. [PubMed]
- Pennock GK, Chow LQ. The Evolving Role of Immune Checkpoint Inhibitors in Cancer Treatment. Oncologist 2015;20:812-22. [PubMed]
- Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011;29:1949-55. [PubMed]
- Azimi F, Scolyer RA, Rumcheva P, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol 2012;30:2678-83. [PubMed]
- Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003;348:203-13. [PubMed]
- Ogino S, Nosho K, Irahara N, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res 2009;15:6412-20. [PubMed]
- Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013;31:860-7. [PubMed]
- Hamid O, Schmidt H, Nissan A, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med 2011;9:204. [PubMed]
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013;14:1014-22. [PubMed]
- Palucka K, Ueno H, Fay J, et al. Dendritic cells and immunity against cancer. J Intern Med 2011;269:64-73. [PubMed]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol 2005;23:515-48. [PubMed]
- Nirschl CJ, Drake CG. Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clin Cancer Res 2013;19:4917-24. [PubMed]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011;331:1565-70. [PubMed]
- Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol 2006;90:1-50. [PubMed]
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol 1996;14:233-58. [PubMed]
- Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev 2009;229:12-26. [PubMed]
- Riley JL, Mao M, Kobayashi S, et al. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci U S A 2002;99:11790-5. [PubMed]
- Schneider H, Downey J, Smith A, et al. Reversal of the TCR stop signal by CTLA-4. Science 2006;313:1972-5. [PubMed]
- Chuang E, Fisher TS, Morgan RW, et al. The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity 2000;13:313-22. [PubMed]
- Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 2005;25:9543-53. [PubMed]
- Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992;11:3887-95. [PubMed]
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027-34. [PubMed]
- Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 2006;203:883-95. [PubMed]
- Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793-800. [PubMed]
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. [PubMed]
- Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999;5:1365-9. [PubMed]
- Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001;2:261-8. [PubMed]
- Chemnitz JM, Parry RV, Nichols KE, et al. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 2004;173:945-54. [PubMed]
- Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med 2012;209:1201-17. [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [PubMed]
- Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2004-12. [PubMed]
- Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [PubMed]
- Butte MJ, Keir ME, Phamduy TB, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007;27:111-22. [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [PubMed]
- Brahmer JR, Rizvi NA, Lutzky J, et al. Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC. J Clin Oncol 2014;32:5s.
- Spigel DR, Gettinger SN, Horn L, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC). J Clin Oncol 2013;31:abstr 8008.
- Soria JC, Cruz C, Bahleda R, et al. Clinical activity, safety, and biomarkers of PD-L1 blockade in non-small cell lung cancer (NSCLC): Additional analyses from a clinical study of the engineered antibody MPDL3280A (anti-PDL1). European J Cancer 2013;49:abstr 3408.
- Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014;20:5064-74. [PubMed]
- Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 2007;13:84-8. [PubMed]
- Marzec M, Zhang Q, Goradia A, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad Sci U S A 2008;105:20852-7. [PubMed]
- Atefi M, Avramis E, Lassen A, et al. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res 2014;20:3446-57. [PubMed]
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [PubMed]
- Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568-71. [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [PubMed]
- Chen L, Gibbons DL, Goswami S, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun 2014;5:5241. [PubMed]
- Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res 2007;13:5262-70. [PubMed]
- Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov 2012;11:790-811. [PubMed]
- Derynck R, Miyazono K, editors. The TGF-β Family. New York: Cold Spring Harbor Laboratory Press, 2008.
- Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003;113:685-700. [PubMed]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003;425:577-84. [PubMed]
- Massagué J. TGFbeta in Cancer. Cell 2008;134:215-30. [PubMed]
- Hawinkels LJ, Ten Dijke P. Exploring anti-TGF-β therapies in cancer and fibrosis. Growth Factors 2011;29:140-52. [PubMed]
- Harradine KA, Akhurst RJ. Mutations of TGFbeta signaling molecules in human disease. Ann Med 2006;38:403-14. [PubMed]
- Flavell RA, Sanjabi S, Wrzesinski SH, et al. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol 2010;10:554-67. [PubMed]
- Erdag G, Schaefer JT, Smolkin ME, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res 2012;72:1070-80. [PubMed]
- Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med 2001;7:1118-22. [PubMed]
- McKarns SC, Schwartz RH, Kaminski NE. Smad3 is essential for TGF-beta 1 to suppress IL-2 production and TCR-induced proliferation, but not IL-2-induced proliferation. J Immunol 2004;172:4275-84. [PubMed]
- Frederick JP, Liberati NT, Waddell DS, et al. Transforming growth factor beta-mediated transcriptional repression of c-myc is dependent on direct binding of Smad3 to a novel repressive Smad binding element. Mol Cell Biol 2004;24:2546-59. [PubMed]
- Thomas DA, Massagué J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 2005;8:369-80. [PubMed]
- Stephen TL, Rutkowski MR, Allegrezza MJ, et al. Transforming growth factor β-mediated suppression of antitumor T cells requires FoxP1 transcription factor expression. Immunity 2014;41:427-39. [PubMed]
- Beyer M, Schultze JL. Regulatory T cells in cancer. Blood 2006;108:804-11. [PubMed]
- Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 2009;30:626-35. [PubMed]
- Peterson RA. Regulatory T-cells: diverse phenotypes integral to immune homeostasis and suppression. Toxicol Pathol 2012;40:186-204. [PubMed]
- Hori S, Nomura T, Sakaguchi S., et al. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299:1057-61. [PubMed]
- Lim HW, Broxmeyer HE, Kim CH. Regulation of trafficking receptor expression in human forkhead box P3+ regulatory T cells. J Immunol 2006;177:840-51. [PubMed]
- Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 2009;30:636-45. [PubMed]
- Hadaschik EN, Enk AH. TGF-β1-induced regulatory T cells. Hum Immunol 2015;76:561-4. [PubMed]
- Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 2003;198:1875-86. [PubMed]
- Tone Y, Furuuchi K, Kojima Y, et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol 2008;9:194-202. [PubMed]
- Maruyama T, Li J, Vaque JP, et al. Control of the differentiation of regulatory T cells and T(H)17 cells by the DNA-binding inhibitor Id3. Nat Immunol 2011;12:86-95. [PubMed]
- Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004;10:942-9. [PubMed]
- Merlo A, Casalini P, Carcangiu ML, et al. FOXP3 expression and overall survival in breast cancer. J Clin Oncol 2009;27:1746-52. [PubMed]
- Petersen RP, Campa MJ, Sperlazza J, et al. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer 2006;107:2866-72. [PubMed]
- Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 2006;441:231-4. [PubMed]
- Gutcher I, Donkor MK, Ma Q, et al. Autocrine transforming growth factor-β1 promotes in vivo Th17 cell differentiation. Immunity 2011;34:396-408. [PubMed]
- Ghoreschi K, Laurence A, Yang XP, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature 2010;467:967-71. [PubMed]
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature 2007;449:419-26. [PubMed]
- Bekeredjian-Ding I, Schäfer M, Hartmann E, et al. Tumour-derived prostaglandin E and transforming growth factor-beta synergize to inhibit plasmacytoid dendritic cell-derived interferon-alpha. Immunology 2009;128:439-50. [PubMed]
- Darrasse-Jèze G, Deroubaix S, Mouquet H, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med 2009;206:1853-62. [PubMed]
- Luo X, Tarbell KV, Yang H, et al. Dendritic cells with TGF-beta1 differentiate naive CD4+CD25- T cells into islet-protective Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A 2007;104:2821-6. [PubMed]
- Dumitriu IE, Dunbar DR, Howie SE, et al. Human dendritic cells produce TGF-beta 1 under the influence of lung carcinoma cells and prime the differentiation of CD4+CD25+Foxp3+ regulatory T cells. J Immunol 2009;182:2795-807. [PubMed]
- Ni XY, Sui HX, Liu Y, et al. TGF-β of lung cancer microenvironment upregulates B7H1 and GITRL expression in dendritic cells and is associated with regulatory T cell generation. Oncol Rep 2012;28:615-21. [PubMed]
- Song S, Yuan P, Wu H, et al. Dendritic cells with an increased PD-L1 by TGF-β induce T cell anergy for the cytotoxicity of hepatocellular carcinoma cells. Int Immunopharmacol 2014;20:117-23. [PubMed]
- Laouar Y, Sutterwala FS, Gorelik L, et al. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol 2005;6:600-7. [PubMed]
- Castriconi R, Cantoni C, Della Chiesa M, et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci U S A 2003;100:4120-5. [PubMed]
- Lee JC, Lee KM, Kim DW, et al. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol 2004;172:7335-40. [PubMed]
- Mantovani A, Sica A, Allavena P, et al. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol 2009;70:325-30. [PubMed]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008;8:958-69. [PubMed]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 2014;6:13. [PubMed]
- Byrne SN, Knox MC, Halliday GM. TGFbeta is responsible for skin tumour infiltration by macrophages enabling the tumours to escape immune destruction. Immunol Cell Biol 2008;86:92-7. [PubMed]
- Smith WB, Noack L, Khew-Goodall Y, et al. Transforming growth factor-beta 1 inhibits the production of IL-8 and the transmigration of neutrophils through activated endothelium. J Immunol 1996;157:360-8. [PubMed]
- Shen L, Smith JM, Shen Z, et al. Inhibition of human neutrophil degranulation by transforming growth factor-beta1. Clin Exp Immunol 2007;149:155-61. [PubMed]
- Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009;16:183-94. [PubMed]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009;9:162-74. [PubMed]
- Srivastava MK, Andersson Å, Zhu L, et al. Myeloid suppressor cells and immune modulation in lung cancer. Immunotherapy 2012;4:291-304. [PubMed]
- Yang L, DeBusk LM, Fukuda K, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 2004;6:409-21. [PubMed]
- Serafini P. Myeloid derived suppressor cells in physiological and pathological conditions: the good, the bad, and the ugly. Immunol Res 2013;57:172-84. [PubMed]
- Levy L, Hill CS. Alterations in components of the TGF-beta superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev 2006;17:41-58. [PubMed]
- Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci U S A 2003;100:8621-3. [PubMed]
- Tang B, Vu M, Booker T, et al. TGF-beta switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J Clin Invest 2003;112:1116-24. [PubMed]
- Wakefield LM, Roberts AB. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev 2002;12:22-9. [PubMed]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002;2:442-54. [PubMed]
- Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008;133:704-15. [PubMed]
- Lou Y, Diao L, Byers LA, et al. Association of epithelial-mesenchymal transition status with PD1/PDL1 expression and a distinct immunophenotype in non-small cell lung cancer: Implications for immunotherapy biomarkers. J Clin Oncol 2014;32:5s.
- Hasmim M, Noman MZ, Lauriol J, et al. Hypoxia-dependent inhibition of tumor cell susceptibility to CTL-mediated lysis involves NANOG induction in target cells. J Immunol 2011;187:4031-9. [PubMed]
- Hasmim M, Noman MZ, Messai Y, et al. Cutting edge: Hypoxia-induced Nanog favors the intratumoral infiltration of regulatory T cells and macrophages via direct regulation of TGF-β1. J Immunol 2013;191:5802-6. [PubMed]


