Prognostic value of circulating endothelial cells in non-small cell lung cancer patients: a systematic review and meta-analysis
Introduction
Lung cancer is one of the most common cancers and accounts for more deaths than any other cancer in both men and women (1). Survival rates for lung cancer remain critically low, the 5-year survival rate for men with lung cancer is 14% compared with 19% for women (2). Current research into lung cancer is mainly focused on noninvasive molecular targets which are used to predict the therapeutic effect of lung cancer treatment and the prognosis of patients with lung cancer.
Circulating endothelial cells (CECs) are mature viable cells that are shed from the endothelium, circulate within the bloodstream and still exhibit proliferative capacity despite their terminal differentiation (3). An alteration in CECs has been associated with several diseases, such as acute coronary syndromes (4), hereditary hemorrhagic telangiectasia (5), anti-neutrophil cytoplasmic antibodies (ANCA) associated vasculitis (6), etc. The hypothesis (7) that tumor growth is angiogenesis dependent was initially proposed in 1971 based on the observation that the expansion of a tumor mass was limited in the absence of angiogenesis. Subsequently, a large amount of experimental data substantiated the hypothesis. In 2001 Mancuso et al. (8) detected increased amounts of CECs in breast cancer for the first time using flow cytometry (FCM). Up to now, CECs have been put forward as promising biomarkers for diagnosis and prognosis of different types of cancers, including, but not limited to, colorectal cancer (9), prostate cancer (10) and pancreatic carcinoma (11).
In 2014 Mehran et al. found that levels of CECs were higher in mice with tumors than without tumors. Then Mehran found the same phenomenon in clinical lung cancer patients compared with normal healthy volunteers (12), so Mehran suggested that CECs are more numerous in cancer patients than in healthy subjects. Soon thereafter several studies supported their conclusion (12-14). However to-date, the relationship between the level of CECs and the diagnosis of non-small cell lung cancer (NSCLC) has had no definitive study. Moreover, the high level of CECs and the change of CECs (∆CECs) have been reported to be correlated with both poor or good prognosis, partially since a single study might be insufficient to detect a possible small effect of CECs and ∆CECs level on NSCLC prognosis, especially when the sample size is statistically small. In this study, a systematic review and meta-analysis to estimate the effect of CECs and ∆CECs level on the survival of NSCLC patients were conducted. It was planned to analyze the prognostic value from the two aspects of progression-free survival (PFS) and overall survival (OS) in patients with NSCLC.
Methodology
Publication search
The electronic databases of PubMed and Web of Science were searched for relevant article from the date of the publications inception until April 30, 2015. The keyword used for the search strategy was “circulating endothelial cells”. A subsequent, more refined search included the terms “cancer” OR “tumor”. Further filter conditions included: (I) only complete studies (abstracts only were excluded) in English; and (II) reports necessary to extract the required indicators. This study was planned, conducted, and reported in adherence to the professional standards of quality for reporting meta-analyses (15).
Inclusion criteria
Studies included in this meta-analysis had to meet all the following criteria: (I) all the patients were diagnosed with NSCLC by histopathology; (II) the ability to evaluate the correlation between CECs or ∆CECs levels and prognosis of NSCLC patients including PFS or OS; and (III) the study directly reported the hazard ratios (HRs) and their 95% confidence intervals (CIs), or provided sufficient information allowing for approximations of HRs and the 95% CIs. If multiple publications from the same study population were available, the most recent and detailed study was eligible for inclusion in the meta-analysis.
Data extraction
Each article was reviewed independently by two authors: Liu Y and Ye W. If there was a difference of opinion, the articles were discussed with a third author: Yuan D. The general data collected from each study were the first author’s name, study country, publication year, type of study, number of patients, therapy schedule, methodology of CECs collection, cut-off value, points of the collection, the cell surface markers, and HR with 95% CI, which were used to measure the effective value.
Quality assessment
The quality of each study was assessed by the same two investigators using “The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses” (16). Given the variability in the quality of related studies searched in the initial literature search, these studies were considered to be high quality if the scores achieved a level of six or more.
Statistical methods
The level of CECs or ∆CECs was considered “high” or “low” according to the cutoff values used in each study. The association between CECs or ∆CECs level and the clinical outcomes was evaluated using the HR of high CECs or ∆CECs level patients over low CECs or ∆CECs level patients and their 95% CI, the HR <1 and 95% CI that did not overlap with 1 implied a good prognosis for the patient with a high level, while the HR >1 and 95% CI that did not overlap with 1 signified poor survival. When these statistical variables were described in text or tables, we obtained them directly from each trial publication. If the data were not given directly, the available data were calculated from Kaplan-Meier curves (using Engauge Digitizer version 4.1) and the HR and 95% CI were calculated using the methods reported by Tierney et al. (17). The Mantel-Haenszel test was used to test significance, and P<0.05 was considered statistically significant. Statistical heterogeneity was assessed by the visual inspection of forest plots, by performing the χ2 test, and by calculating the I2 value. Heterogeneity was considered significant if the P value is less than 0.1. The value of I2 is used to assess the degree of heterogeneity (I2<25% no heterogeneity; I2=25-50% moderate heterogeneity; I2>50% large or extreme heterogeneity).
Publication bias evaluation
Potential publication bias was evaluated using Begg’s test (18) and Egger’s test (19) and standard error was plotted against log (HR) to form a simple scatterplot. P<0.05 was considered to be representative of a significant statistical publication bias. All of the statistical analyses were performed with STATA 11.0 (StataCorp, College Station, TX, USA). All P values were two tailed.
Results
Study selection and characteristics
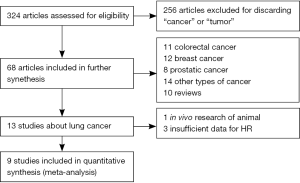
Our initial search identified 324 citations from a search of the above databases using the search strategy as previously described. Two hundred and fifty-six reports were excluded as they were not research about cancers or tumors when we scanned the abstracts. Subsequently, in the remaining 68 research papers, included 11 colorectal cancer, 12 breast cancer, 8 prostatic cancer, 10 reviews, and 14 other cancer papers and finally 13 reports were about lung cancer. After in depth analysis of these 13 reports, one report focused on animal experiment and three reports did not provided sufficient data about HR, thus there additional four reports were excluded from the selection. Thus, nine reports fit the selection criteria for this research (Figure 1), including eight prospective studies and one retrospective study.
The clinical characteristics of these nine included studies eligible for the meta-analysis are summarized in Table 1; two studies evaluated patients from Spain, one from Japan, two from Syria, one from France, and three from China. They were primarily published between 2009 and 2015 (Table 1). The nine studies, which involved 772 NSCLC patients, with the sample sizes ranging from 31 to 151 patients (mean 85.8). Two hundred and fifty-seven patients were not accepted into the sample population or were lost during follow-up before the disease progressed. The number completing the entire survey was 515.

Full table
Therapy schedule and sample collection
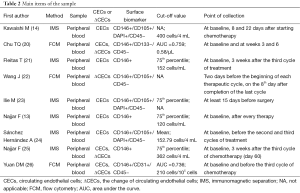
The standard courses of treatment were double-agent platinum-based chemotherapy (14,24,25), chemotherapy combined with anti-angiogenesis (20-22,26), chemotherapy combined with radiotherapy (13), and surgery (23). All studies obtained the peripheral blood (PB) to separate the CECs in NSCLC patients. The points of collection and the cut-off value were different for each study (Table 2). In addition, HR values in two datasets were directly extracted from original data, and seven were extrapolated from survival curves.
Full table
Baseline CECs counts and PFS in NSCLC patients
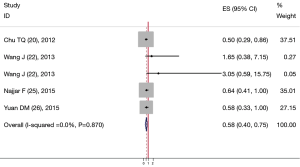
Six studies including 387 patients reported data on baseline CECs counts and PFS in NSCLC. One report (14) implied that patients who had a higher baseline CECs count possessed a longer PFS. However, one study (23) suggested that a high CECs count at baseline significantly correlated with shorter PFS. The balance of the four studies (13,24-26) insisted that there was no significant correlation between baseline CECs count and PFS. Finally, combined data from all six studies suggested that high level of baseline CECs counts and long PFS had a positive relationship with a pooled HR estimate of 0.71 (95% CI: 0.529-0.891) (Figure 2). The heterogeneity between the studies was not significant (I2=21.2%, P=0.274) in either analysis.

∆CECs levels and PFS in NSCLC patients
Four studies including 273 patients with NSCLC, reported data on the change of CECs after therapy. One study was categorized randomly into two segments undergoing separate regimens of chemotherapy with Rh-endostatin or single chemotherapy, and followed-up to disease progression, respectively. So, five related studies were included about ∆CECs level and PFS. Three related studies (20,25,26) maintained that greater reduction of CECs significantly correlated with longer median PFS, the other related study (22) indicated that there was no correlation between ∆CECs level and PFS. Combined data from these studies showed patients with a high percentage change in CECs counts after therapy had significantly longer PFS than those with low percentage change. The pooled HR estimate was 0.575 (95% CI: 0.401-0.75) (Figure 3). As well, there was no heterogeneity between the reports with I2=0.00%, P=0.742.
Baseline CECs counts and OS in NSCLC patients
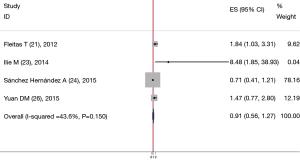
In total, four reports were involved in baseline CECs counts and OS in patients with NSCLC. In the context of a single group, two (21,23) of these studies with high CECs counts at baseline had a significantly worse OS, with a P value of 0.006 and 0.04, respectively. The other two experiments did not find a correlation between CECs baseline levels and the OS in patients with NSCLC; however, when these studies which had been analyzed were merged with each other; the combined HR was 0.914, 95% CI was 0.560-1.267. And there was only small heterogeneity (I2=43.6%, P=0.150) (Figure 4).
Publication bias
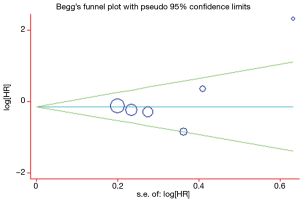
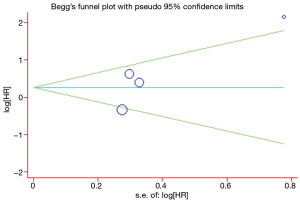
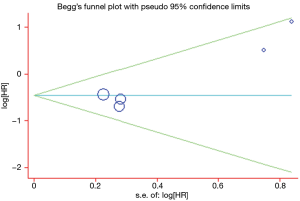
A Begg’s funnel plot was prepared for the visual assessment of obvious publication bias for the included studies in CECs or ∆CECs levels. There was no overt evidence of significant publication bias for the studies included in our meta-analysis by the P value (PCECs-PFS=0.462; P∆CECs-PFS=0.221 and PCECs-OS=0.308). An Egger’s test was then employed for the formal evaluation and statistical significance was deemed when the P<0.05. The PCECs-PFS=0.177 (Figure 5) and the PCECs-OS=0.210 (Figure 6), indicated that there were no significant publication bias among these studies, which concerned the base CECs counts of the PFS or OS in NSCLC patients. Alternatively, there did exist statistical differences on publication bias between the studies on the change of CECs after therapy and PFS, as the P∆CECs-PFS=0.043 (Figure 7).
Discussion
In recent years, personalized pharmacotherapy was more and more popular in oncology. Some researchers had examined the major subtype of driver mutations that have been identified in NSCLC and summarized the relevant therapies, in order to contribute to the personalized treatment (27). After the diagnosis and treatment, increasing attention is being paid to the prognostic biomarkers in NSCLC patients, such as Ki67, carcinoembryonic antigen (CEA), neuron-specific enolase (NSE) etc. CEC is a new prediction of these people. Generally speaking, in healthy donor’s PB can also detect the existence of the CECs, but the level is very low with typical counts being 0-20 cells per ml of blood (28). The present meta-analysis is the first study to systematically evaluate the association between CECs or ∆CECs and prognosis in NSCLC patients. Our study combined the outcomes of 515 NSCLC patients from nine reports, which revealed a longer PFS in NSCLC patients having higher levels of baseline CECs and ∆CECs. Although, the base level of CECs has no effect on OS, Chu et al. (20) and Yuan et al. (26) showed that the median survival of patients with greater CECs reduction was significantly longer than those with lower reductions. As these indexes were inconformity in each research, collocated and correlated data was not further analyzed.
Originally three techniques are being used to detect CECs, including reverse transcription-polymerase chain reaction (RT-PCR), immunomagnetic separation (IMS) and FCM (29), but as preclinical data is conflicting, using RT-PCR to quantify CECs has been largely abandoned (30), so the latter two are the commonly used method. Similarly, the IMS and FCM depend on CD146 driven immunomagnetic isolation. At times, further identification also needs the expression of additional endothelial markers such as 2-(4-amidinophenyl)-1 Hindole-6-carboxam- idine (DAPI) or CD105 (31). This meta-analysis included studies using IMS or FCM to count CECs, in these studies with CD146 being the primary positive marker on the CECs surface along with other morphological characteristics comprising CD105+, DAPI+, CD31+, CD133− or CD45−, reference to Table 2. Method and the choice of surface markers result in the different measuring values, this will bring different cut-off values. In these nine studies, the basis of cut-off values included the mean, the 75th percentile, the optimal area under receiver operating characteristic (ROC) curve and two entire studies had no mention of the terms. Cut-off value of research directly influence the grouping and the outcome.
Angiogenesis is considered to be an absolute prerequisite for malignant tumour growth and metastasis (32). Because the incomplete basement membrane, tumor blood vessels are immature and are therefore prone to rupture (33). So, anti-angiogenesis therapy has emerged. Since the first vascular endothelial growth factor (VEGF)-targeting drug (bevacizumab) was approved by the US Food and Drug Administration in 2004, the anti-angiogenic drugs have proliferated rapidly. Despite anti-angiogenic drugs prolonging the survival of patients with lung, colon, and renal carcinomas (34-36). Four reports involving CECs counts and anti-angiogenesis therapy (20-22,26) are included in this meta-analysis. The anti-angiogenesis drugs include Rh-endostatin and bevacizumab, the related data demonstrated that the CECs counts of the anti-angiogensis therapy group decreased significantly compared with the control group. Moreover, in the majority of studies, it was found that CECs levels significantly decreased after chemotherapy in partial response (PR) patients, with contrasting results being obtained in progressive disease (PD) patients (14,20,22,24-26). The other study implied that the amounts of CECs at baseline in the patients who showed PD were significantly lower than in PR patients (14). This revealed that the CECs counts appeared to be a promising predictive maker of the clinical efficacy of chemotherapy, especially combined with anti-angiogensis therapy.
In addition, one study was collected from preoperative blood samples from 74 patients who underwent resection for NSCLC (23), the data displayed high levels of CECs at baseline significantly correlated with shorter PFS and OS (P<0.001, P=0.005, respectively) of NSCLC patients. This is contrary to other studies, temporary surgical stimulation might affect the vascular system disorder, or the duration of follow-up can also affect this.
As far as the data is concerned, no meta-analysis regarding this relationship has been published to-date. Previous studies have reported inconsistent and conflicting results about the association between the CECs counts at baseline or the change of CECs levels after therapy and the prognosis of NSCLC. The limitations in size and follow-up proved to be insufficient in one individual study, this meta-analysis made up for this defect to a certain extent. Nevertheless, several limitations of this meta-analysis should be indicated. First, in terms of meta-analysis, some inevitable publication bias may exist, because the failure of researchers to submit negative studies for publication, or negative results are often rejected by journals, whereas positive results tend to be accepted (37). Second, since some HRs were not directly reported in the studies, it was necessary to calculate HR from the data provided in the papers or extrapolate them from the survival curves. The estimated HR might be less reliable than the data obtained directly from published statistics. Third, the patient’s general conditions of these studies are also the source of bias, such as race, age, complication, the sensitivity of the response to drugs and so on. Therefore, further high quality studies are needed, which include a large number of incident NSCLC cases with sufficient follow-up time, and information available on all patients and the relationship between CECs counts and the diagnosis or OS of the NSCLC.
Conclusions
In summary, results of our meta-analysis suggest that CECs counts at baseline and ∆CECs levels could be used to determine the prognosis of patients with NSCLC and predict the effectiveness of the treatment without the limitations; especially it might be more ideal than the baseline CECs counts as a prognostic factor in patients with NSCLC that the reduction of CECs after therapy.
Acknowledgements
Funding: This study is supported by the Foundation of Jiangsu Lung cancer diagnostic and treatment research in Medical Science of China (BL2013026). The National Natural Science Foundation of China (No. 81572273) and the National Natural Science Foundation of China (No. 81572937).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008 v1.2: Cancer incidence and mortality worldwide: IARC CancerBase No. 10. Lyon: IARC, 2010.
- Howlader N, Noone AM, Krapcho M, et al. editors. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations). Bethesda, MD: National Cancer Institute; 2012. Available online: http://seer.cancer.gov/archive/csr/1975_2009_pops09/
- Beerepoot LV, Mehra N, Vermaat JS, et al. Increased levels of viable circulating endothelial cells are an indicator of progressive disease in cancer patients. Ann Oncol 2004;15:139-45. [PubMed]
- Schmidt DE, Manca M, Hoefer IE. Circulating endothelial cells in coronary artery disease and acute coronary syndrome. Trends Cardiovasc Med 2015;25:578-87. [PubMed]
- Massa M, Canzonieri C, Campanelli R, et al. Increase of circulating endothelial cells in patients with Hereditary Hemorrhagic Telangiectasia. Int J Hematol 2015;101:23-31. [PubMed]
- Woywodt A, Streiber F, de Groot K, et al. Circulating endothelial cells as markers for ANCA-associated small-vessel vasculitis. Lancet 2003;361:206-10. [PubMed]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971;285:1182-6. [PubMed]
- Mancuso P, Burlini A, Pruneri G, et al. Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood 2001;97:3658-61. [PubMed]
- Manzoni M, Comolli G, Torchio M, et al. Circulating endothelial cells and their subpopulations: role as predictive biomarkers in antiangiogenic therapy for colorectal cancer. Clin Colorectal Cancer 2015;14:11-7. [PubMed]
- Bertolini F, Shaked Y, Mancuso P. On the clinical relevance of circulating endothelial cells and platelets in prostate cancer. Br J Cancer 2013;108:1387. [PubMed]
- Kondo S, Ueno H, Hashimoto J, et al. Circulating endothelial cells and other angiogenesis factors in pancreatic carcinoma patients receiving gemcitabine chemotherapy. BMC Cancer 2012;12:268. [PubMed]
- Mehran R, Nilsson M, Khajavi M, et al. Tumor endothelial markers define novel subsets of cancer-specific circulating endothelial cells associated with antitumor efficacy. Cancer Res 2014;74:2731-41. [PubMed]
- Najjar F, Alammar M, Bachour M, et al. Circulating endothelial cells as a biomarker in non-small cell lung cancer patients: correlation with clinical outcome. Int J Biol Markers 2014;29:e337-44. [PubMed]
- Kawaishi M, Fujiwara Y, Fukui T, et al. Circulating endothelial cells in non-small cell lung cancer patients treated with carboplatin and paclitaxel. J Thorac Oncol 2009;4:208-13. [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9, W64.
- Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [PubMed]
- Chu TQ, Ding H, Garfield DH, et al. Can determination of circulating endothelial cells and serum caspase-cleaved CK18 predict for response and survival in patients with advanced non-small-cell lung cancer receiving endostatin and paclitaxel-carboplatin chemotherapy? A retrospective study. J Thorac Oncol 2012;7:1781-9. [PubMed]
- Fleitas T, Martínez-Sales V, Vila V, et al. Circulating endothelial cells and microparticles as prognostic markers in advanced non-small cell lung cancer. PLoS One 2012;7:e47365. [PubMed]
- Wang J, Xiao J, Wei X, et al. Circulating endothelial cells and tumor blood volume as predictors in lung cancer. Cancer Sci 2013;104:445-52. [PubMed]
- Ilie M, Long E, Hofman V, et al. Clinical value of circulating endothelial cells and of soluble CD146 levels in patients undergoing surgery for non-small cell lung cancer. Br J Cancer 2014;110:1236-43. [PubMed]
- Sánchez Hernández A, José Juan O, Vidal Martínez J, et al. Quantification of circulating endothelial cells as a predictor of response to chemotherapy with platinum and pemetrexed in patients with advanced non-squamous non-small cell lung carcinoma. Clin Transl Oncol 2015;17:281-8. [PubMed]
- Najjar F, Alammar M, Bachour M, et al. Predictive and prognostic value of circulating endothelial cells in non-small cell lung cancer patients treated with standard chemotherapy. J Cancer Res Clin Oncol 2015;141:119-25. [PubMed]
- Yuan DM, Zhang Q, Lv YL, et al. Predictive and prognostic significance of circulating endothelial cells in advanced non-small cell lung cancer patients. Tumour Biol 2015. [Epub ahead of print]. [PubMed]
- Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res 2015;4:36-54. [PubMed]
- Rowand JL, Martin G, Doyle GV, et al. Endothelial cells in peripheral blood of healthy subjects and patients with metastatic carcinomas. Cytometry A 2007;71:105-13. [PubMed]
- Strijbos MH. Circulating tumour cells and circulating endothelial cells as biomarkers in oncology. Acta Clin Belg 2011;66:332-6. [PubMed]
- Strijbos MH, van Krimpen BA, Debets R, et al. mRNA levels of CD31, CD144, CD146 and von Willebrand factor do not serve as surrogate markers for circulating endothelial cells. Thromb Haemost 2010;104:318-26. [PubMed]
- Woywodt A, Blann AD, Kirsch T, et al. Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: proposal of a definition and a consensus protocol. J Thromb Haemost 2006;4:671-7. [PubMed]
- Kerbel RS. Tumor angiogenesis. N Engl J Med 2008;358:2039-49. [PubMed]
- Popper HH, Ryska A, Tímár J, et al. Molecular testing in lung cancer in the era of precision medicine. Transl Lung Cancer Res 2014;3:291-300. [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [PubMed]
- Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004;22:2184-91. [PubMed]
- Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003;349:427-34. [PubMed]
- Dickersin K, Min YI. Publication bias: the problem that won’t go away. Ann N Y Acad Sci 1993;703:135-46; discussion 146-8. [PubMed]