MET genetic lesions in non-small-cell lung cancer: pharmacological and clinical implications
Lung cancer is the leading cause of death for solid tumors worldwide with an annual mortality of over one million. Lung carcinoma includes a series of different diseases which are roughly divided into two groups based on clinical and histo-pathological features: non-small cell lung cancer (NSCLC), accounting for almost 80% of lung cancer diagnosis and small cell lung cancer (SCLC) responsible for the remaining 20%. The NSCLC molecular profile has been deeply investigated; alterations in several oncogenes, tumor suppressor genes and transcription factors have been detected, mainly in adenocarcinomas. Dissection of such a complex scenario represents a still open challenge for both researchers and clinicians. MET, the receptor for Hepatocyte Growth Factor (HGF), has been recently identified as a novel promising target in several human malignancies, including NSCLC. Deregulation of the HGF/MET signaling pathway can occur via different mechanisms, including HGF and/or MET overexpression, MET gene amplification, mutations or rearrangements. While the role of MET mutations in NSCLC is not yet fully understood, MET amplification emerged as a critical event in driving cell survival, with preclinical data suggesting that MET-amplified cell lines are exquisitely sensitive to MET inhibition. True MET amplification, which has been associated with poor prognosis in different retrospective series, is a relatively uncommon event in NSCLC, occurring in 1-7% of unselected cases. Nevertheless, in highly selected cohorts of patients, such as those harboring somatic mutations of EGFR with acquired resistance to EGFR tyrosine kinase inhibitors, MET amplification can be observed in up to 20% of cases. Preclinical data suggested that a treatment approach including a combination of EGFR and MET tyrosine kinases could be an effective strategy in this setting and led to the clinical investigation of multiple MET inhibitors in combination with anti-EGFR agents. Results from ongoing and future trials will clarify the role of anti-MET molecules for the treatment of NSCLC and will provide insights into the most appropriate timing for their use. The present review recapitulates the current knowledge on the role of MET signaling in NSCLC mainly focusing on its implications in molecular diagnostic approach and on the novel targeted inhibitors.
Key words: Lung cancer; translational oncology; target therapy; invasive growth; resistance
Introduction
Lung cancer is the leading cause of death for solid tumors worldwide with an annual mortality of over one million (1). Nevertheless it encompasses heterogeneous diseases which are roughly divided into two groups based on clinical and histo-pathological features: non-small cell lung cancer (NSCLC), accounting for almost 80% of lung cancer diagnosis and small cell lung cancer (SCLC), responsible for the remaining 20%. NSCLCs are further classified as: adenocarcinoma (ADC); squamous cell carcinoma (SCC) and large cell carcinoma (LCC) - comprising the neuro-endocrine variant (LCNEC). Recently the American Thoracic Society and the European Respiratory Society approved a new classification of lung adenocarcinomas (2) which is address to ADCs, now classified by a more adequate multidisciplinary perspective.
The traditional therapeutic management of advanced lung cancer which includes chemotherapy, with or without radiation, is now supplemented with a personalized approach. As molecular mechanisms have been explored in lung cancer more recently, the personalized approach has incorporated cancer molecular abnormalities beyond standard clinical/pathological parameters. Lung cancers are characterized by an extremely diverse collection of genomic alterations and numerous pathogenetically important changes have already been detected in a substantial fraction of patients and translated into a system from detection and determination of the prognosis of the disease.
Among them MET, the receptor for Hepatocyte Growth Factor (HGF) is emerging as involved in the onset and progression of several human malignancies, including lung cancer. The MET oncogene is the key player of the genetic program named ‘invasive growth’, a biological process which leads cells to detach from themselves, scatter, migrate and invade distant sites (3). This process is essential in a wide variety of physiological and pathological settings and is usurped by cancer cells. Several therapeutic approaches that block MET signaling are under investigation and a number of inhibitors have landed the clinical setting. Within respect to lung cancer, several lesions affecting the MET oncogene have been detected, mainly in the context of NSCLC. While the role of MET mutations in lung tumors is not yet fully understood, MET amplification emerged as a critical event in conferring to the neoplastic clone a selective advantage for both malignant progression and drug resistance.
The aim of this review is to analyze the molecular basis of invasive growth activation in lung cancer onset and progression and to review the evidence that indicates MET (the major player of invasive growth) as a promising targetable candidate in lung cancer therapy.
The MET driven invasive growth pathway
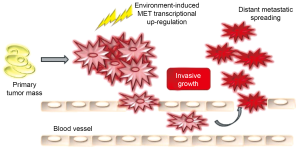
Tissue plasticity is a fundamental feature of multicellular organisms, which is necessary for the progressive acquisition of a functional identity from undifferentiated cells in embryo development and for maintenance of the full integrity of the organisms in adult life. The biological programs that concur in the specification of tissue dynamic changes are orchestrated by the integration of different biological activities, including cell proliferation, survival, cell-cell dissociation (‘scattering’), migration, invasion, and morphogenesis: these events are collectively defined as ‘invasive growth’ (4). Invasive growth is essential in a wide variety of physiological and pathological events. During embryogenesis it drives events such as gastrulation and nervous system development and in adult organisms regulates inflammatory processes and tissue remodeling during wound healing. The pathological counterparts are the biological mechanisms that cause local invasion and metastases. During neoplastic progression, cells proliferate without control, loose contact-inhibition, detach from their primitive sites and invade organs giving rise to secondary colonies. Indeed, from a biological and clinical point of view a tumor is defined as malignant when neoplastic cells acquire the ability to disseminate from their natural context into the lymphatic and blood vessels and colonize tissues and organs that are distant from the original site of growth (5). Morphogenesis and metastases seem to arise from the same genetic program that instructs cells to that biological process named anoikis. Through a mechanism known as epithelial-mesenchymal transition (E.M.T.), cancer cells acquire a metastatic phenotype (6). Metastatic cells reach a secondary site via blood or lymphatic vessels; after extravasation and the arrest of tumor cells in distant organs, the EMT process could be reverted through a mesenchymal-epithelial transition (M.E.T.); this last step coincides with cell repolarization and terminal differentiation in tissue pattern that usually resembles branching tubules (7). Since the new microenvironment of the metastatic site differs from that of primary mass, the cells can die or survive; in case of survival if proliferation is balanced by apoptosis metastases remain clinically undetectable (dormant micrometastases); on the other hand they can undergo another EMT, giving rise to macroscopic lesions that are generally untreatable and can essentially determine the patient’s death.
Several cytokines and growth factors can induce proliferation, differentiation, chemotaxis, migration, protection from apoptosis: for example epidermal growth factor (EGF), insuline growth factor-1 (IGF-1), fibroblast growth factor (FGF). However a family of soluble growth factors plays a central role in mediating normal and neoplastic invasive growth in epithelial cells: they are known as scatter factors (8), represented mainly by Hepatocyte Growth Factor (HGF) and Macrophage Stimulating Protein (MSP) and their receptors (the tyrosine-kinase receptors codified by MET and RON genes); data are emerging that two other families of molecules that are structurally related to MET are probably involved in this program: semaphorins (that act as ligands) and plexins (that act as receptors).
HGF was identified independently as a platelet-derived mitogen for hepatocytes (9) and as a fibroblast-derived factor that is capable of inducing epithelial cell scattering, meaning cell dissociation and motility (10); later HGF and scatter factor were found to be the same molecule (11).
It has been demonstrated that scattered phenotype induced by HGF is equivalent to EMT, at least in defined cellular models (12); HGF is also a potent angiogenic factor. In vivo mice carrying homozygous mutations of HGF or MET genes die in utero because of several defects during placental and liver development (13,14) and are devoid of muscles that derive from the migratory myogenic precursors that detached from the myotome (15).
Scatter factors HGF and MSP belong to the plasminogen family of proteins. Plasminogen-family members are defined by: (I) the presence of at least one characteristic domain known as kringle (an eighty aminoacid double-looped structure that is formed by three internal disulphide bridges); (II) a serine protease domain; (III) an activation segment that is located between the kringle and the protease domains. Among the members of this family HGF and MSP are unique in the sense that that they lack proteolytic activity; owing to replacement of the histidine and serine residues that are contained within the catalytic site of serine proteases with glutamine and tyrosine, respectively. Scatter factors are secreted as single-chain, biologically inert glycoprotein precursors and are converted into their bioactive form in the extracellular environment by specific proteases, which break the bond between two positively charged aminoacids (dibasic site Arg494- Val495). The mature factors are heterodimers that consist of an α- and a β-chain held together: the α-chain contains the hairpin loop of about 27 aminoacids (homologous to the pre-activation peptide of plasminogen) followed by four kringles and the β-chain that contains the serine protease-like structure (3).
As describe above, during cancer progression, neoplastic cells must first suppress the adhesive strength that maintains the normal compaction of epithelia and their in situ multicellular organization: they must reorganize their integrin repertoire to impair contacts with the underlying basement membrane, which separate them from the surrounding connective tissue. So cells must create proteolytic defects in membrane basement and stromal matrix to make openings for their migration, and they will eventually reach the circulation. In epithelial cells intracellular adhesion is primarily mediated by adherence junctions: in these sites physical association between neighboring cells is mediated by transmembrane cadherins which are connected to cytoskeleton by catenins, which forms a submembraneous scaffold that structurally and functionally links cadherins to the actin microfilaments (16). HGF triggers destabilization of adherence junctions by transcriptional downregulation of cadherins (17,18), redistribution of the cadherin-catenin complexes from the actin cytoskeleton to the soluble membrane fraction (19,20). Matrix metalloproteinases (MMPs) mediate proteolytic cleavage of cadherins from the cell surface (21) or disruption of the structural integrity of adherens junctions by catenin tyrosine phosphorylation (22): in all cases HGF weakens aggregation of carcinoma cells and facilitates dissociation and scattering. When cells that have detached from the primary tumor invade surrounding tissues and elude apoptotic program, which normally occurs when cells are placed in abnormal contexts, the neoplastic clone acquires the ability to recognize the modified extracellular environment by displaying a versatile set of adhesive receptors, by either expression of new integrins or functional activation of dormant integrins. HGF can strongly influence this cellular strategy both by upregulating integrin transcription and by stimulating the aggregation at actin-rich adhesive sites (23,24): this modulation allows cancer cells to survive and migrate within stromal context that, in physiological conditions, would lead cells to death. By interacting with some integrins at the edge of migrating neoplastic clone, MMPs act localizing and converting digestion only at sites of cancer invasion so that to avoid indiscriminate matrix disruption (25). HGF has an important role in regulating MMPs proteolytic activity both enhancing the transcriptional levels of a large number of MMPs and by stimulating conversion of their precursors (26,27). Another crucial step in the process of metastatic dissemination is the availability of accessible sites for intravasation of neoplastic cells. Intratumoral angiogenesis produces a vascular network that is in close contact with neoplastic cells and allows them to penetrate the capillary walls and enter the circulation. From this point of view it has been demonstrated that HGF is a potent angiogenic mediator and stimulates the degrading activity of MMPs, so that neoplastic cells can go through the eroded basement membrane and invade blood and lymphatic stream to reach distant organs (28). The signaling pathway of HGF is mediated by its receptor MET, a transmembrane protein dysplaing tyrosine kinase activity encodeded by the proto-oncogene MET (29).
The MET gene is located on chromosome 7, band q31; it is constituted by 21 exons separated by 20 introns codifying for a trans-membrane tyrosine kinase receptor made of a disulphide-linked heterodimer, which originates after the proteolytic cleavage of a single chain precursor (Figure 1). The heterodimer is formed by a single-pass transmembrane 145 kDa α-chain and an extracellular 50-kDa β-chain. The extracellular region contains a conserved 500 aminoacids SEMA domain, which is involved in ligand-receptor interaction, a cysteine rich domain (Cys domain) made of 80 aminoacids known as MET-related sequence (Mrs) and a protein-protein interaction site made of four immunoglobulin-like structures (IPT domain). The intracellular portion of the receptor is made of a juxtamembrane (JM) region containing the residue S985 which is essential for receptor downregulation and a tyrosine (Tyr 1003) that, upon phosphorylation, it is able to bind the E3-ubiquitin ligase Cbl, which finally promotes receptor ubiquitinization and degradation; a catalytic site which contains two tyrosines (1234 and 1235) which regulate the enzymatic activity and a C-terminal regulatory tail in which there are two tyrosines (1349 and 1356) that when phosphorylated create a unique docking site which is responsible for the recruitment of a wide-spectrum of downstream mediators; it has been previously demonstrated that these two tyrosines are essential and sufficient to confer to the receptor is physiological and oncogenic potential (30,31). Historically MET was identified as the protein product of a transforming oncogene, TPR-MET which was derived from a chromosomal rearrangement in an osteosarcoma cell line treated with a chemical carcinogen (32).
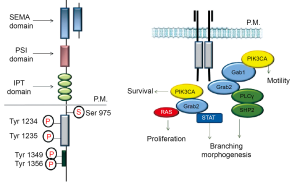
From then on, a large number of in vitro and in vivo studies have demonstrated MET’s tumorigenic activities and have linked this behavior to the deregulation of the catalytic function. Ligation of HGF to MET induces receptor catalytic activity. When the receptor is in an active state its conformation is inaccessible to ATP; once activated by dimerization the activation loop in unlocked by trans-phosphorylation of Tyr 1234 and Tyr 1235 which are essential for enzymatic activity (11). After activation, MET elicits intramolecular phosphorylation of the other two critical tyrosine residues (Tyr 1349 and Tyr 1356), at the C-terminal of the β-chain: these two sites and the surrounding aminoacids constitute the so called multifunctional docking site, a motif that is activated after phosphorylation and mediates interaction with multiple SH2-containing signal transducers, including the cytosolyc tyrosine kinase SRC (33), the lipid kinase phosphatidylinositol 3-phosphate kinase (PI3K) (34), the transcription factor STAT3 (34) and the adaptor proteins GRB2 (34), SHC (35) and Gab1 (36). Gab1 is a high-capacity scaffolding adaptor that sustains MET indirect interaction with additional transducers, such as phospholypase C-γ (PLC-γ) and the protein tyrosine phosphatase SHP2 (37).
Through the activation of these pathways MET receptors induces a series of biological processes that lead to invasive growth (Figure 2). The specificity of this peculiar response is determined by qualitative activation of specific pathways that are responsible for oncogenic and migratory effects of MET. Through pharmacological inhibition of each pathway it has been possible to understand qualitative differences consequent to MET activation: the in vitro results have shown that activation of PI3K alone is sufficient for cell motility (38), the RAS pathway is necessary and sufficient for proliferation (39) activation is necessary for full biological response activation of PLC-γ is required for differentiation and acquisition of cell polarity (40) but is dispensable for cell dissociation, motility, growth; the serine/threonine kinas c-AKT/protein kinase B (PKB) a downstream effector of PI3K is involved in preventing cell anoikis (41). From a quantitative point of view it has been demonstrated that the biological response is related to duration of MET signaling: if in physiological processes MET activation is a transient event, during metastatization MET is constitutively active and lead to prolonged activation of the downstream transducers (42). Quantitative parameters indicate that prolonged tyrosine phosphorylation of Gab1 together with sustained activation of MAPK as required for branching morphogenesis- a long term effect with is specifically induced by scatter factor while a transient peak of MAPK activity is related to pure mitogenic effect (43).
Finally signal specificity may also be related to the cellular context in which it works, meaning that tissue specific membrane molecules provide a preferential docking site for diversification of intracellular signals (44). It has been demonstrated that MET has at least four partner molecules: integrin α6β4 (45), Plexin B1 (46), CD44 (47) and Fas (48).
In conclusion invasive growth brings with it the dilemma of how to dissociate the beneficial trophic properties of scatter factors from their aberrant activation during metastatization. These different effects are related to the unique biological responses generated by MET activation which are the consequences of combination and integration of specific pathways triggered by receptor activation and by the interaction with the environment in which the receptor itself is situated and are supported by the unique structural properties of scatter factors family which contains distinct domains that are independently able to interact with their specific receptors thus influencing cellular response (49-52). Through the activation of these pathways MET receptor induces a series of biological processes that lead to invasive growth. The specificity of this peculiar response is determined by qualitative activation of specific pathways that are responsible for oncogenic and migratory effects of MET. Besides, more recent studies have been focused on the modulation (quantitative activation) of MET signaling. Indeed signal transduction can depend not only by the protein-protein interaction, but also by the levels of MET receptor on the cell surface (mediated for example by spatially restricted events and ubiquitin-mediated degradation) and by the capacity of receptor internalization through endosomal vesicles.
MET genetic lesion in cancers
The morphogenetic properties of the MET receptor for HGF, when displayed in an aberrant cellular context and without temporal regulation, can turn into deleterious effects that are responsible for the onset and progression of malignancies. Under these conditions MET’s growth promoting activity causes cellular transformation whereas its ability to enhance motility and survival accounts for neoplastic invasion and metastases. Constitutive activation of the tyrosine kinase receptors can be reached through three mechanisms: (I) ligand-receptor autocrine circuits - which free cells from the need for a paracrine supply of growth factor; (II) receptor overexpression which favors local receptor oligomerization and reciprocal activation even in the absence of the ligand; (III) structural alterations in the receptor that keep it functioning continuously. MET expression in human cancer is generally driven by overexpression which acts through enhanced transcription and amplification - increased gene copy number (53-55) - and confers to neoplastic cells a selective advantage for malignant progression (56) and drug resistance (57). Mutations are rarely found. MET gene mutations may promote tumorigenesis, such as the germline missense changes found in hereditary papillary renal cell carcinoma. Most frequently missense mutations are located in the tyrosine kinase (TK) domain, although mutations in a number of other domains have been identified. Mutations in the SEMA domain affect binding to HGF, those in the JM domain affect the actin cytoskeleton, cell motility and migration; mutations in the TK domain facilitate oligomerization and activate MET in the absence of HGF (32,58-61). These may be present in both primary tumors and nodal, lung, and liver metastases (53,62).
More often overexpression is consequent to transcriptional up-regulation, without gene amplification and is related to hypoxia, -low oxygen tension in surrounding stroma (63) - and it is induced by ionizing radiation (64) in those cases MET acts as a ‘sensor’ for adverse microenvironmental conditions. A strong amplification of the MET gene has been reported in many human cancers including non-small cell lung cancer (NSCLC) with acquired resistance to epidermal growth factor receptor (EGFR) inhibitors (65). Besides HGF itself is able to transcriptionally induce MET and, since HGF is ubiquitously present through our organism and active in tumor stroma it becomes evident the role of this cytokine as a landscaper factor, meaning that it empowers with positive feedback loop cancer cells disseminations. Interestingly this finding is in agreement with the experimental observation showing that MET activating mutations require HGF to completely activate the catalytic efficiency (66). Notably, MET overexpression may act as a prognostic maker: in NSCLC, MET and HGF levels correlate with prognostic parameters and poor survival (67). Patients with metastatic disease demonstrate higher MET expression at metastatic sites and higher plasma MET levels (64).
Importantly, Boccaccio et al. demonstrated that MET drives a genetic program linking cancer to haemostasis (68). The molecular pathogenesis of this syndrome (Trousseau’s syndrome) is related to the ability of MET to up-regulate the expression of the genes encoding plasminogen activator inhibitor type-1 (PAI-1) and cyclooxygenase 2 (COX-2) supporting the thrombohaemorragic phenotype. This finally results in an advantage for tumor cells because the establishment of a fibrin matrix forms rudimentary scaffold that helps the growth of the tumor itself and the encoming vessels. Taken together these evidences underline the fact that MET activation sustains dissemination of neoplastic emboli by increasing both angiogenesis and the stroma network; besides it has been shown that systemic MET inhibition - on tumor and host cells - is able to retard not only the growth of the MET positive xenografts but also of the tumors cells that do not express MET (69) supporting the hypothesis that MET inhibition can be therapeutic even through the only impairment of angiogenesis. As previously discussed, MET is trascriptionally activated by hypoxia (63), meaning that the more a neoplastic tissue is hypoxic the more is prone to distant dissemination. This fact might be kept in consideration before starting an anti-angiogenetic therapy: targeting angiogenesis in cancer means essentially to cut off tumor’s blood supply and this in turn, con induce MET overexpression. This evidence points out the therapeutic rationale to combine anti-angiogenetic agents - which have been used and tested in lung cancer patients - and MET inhibitors in order to prevent cancer cells spreading consequent to oxygen deprivation. Finally this observation confirms the dual value of MET inhibition in cancer that is directed to block both the invasive potential and the pro-angiogenetic effect of the neoplastic clone.
In conclusion MET is a necessary oncogene for restricted subsets of human cancer, which depend on (‘addiction’) MET aberrant activation for their own growth and survival. Interestingly, in some instances, NSCLC could show a MET addicted phenotype, and in those settings MET inhibition results in a proliferative block or massive death in a way similar to the one described with EGFR inhibitors. In these cancer types the identification of MET amplification can be considered a strong predictor of therapeutic response with anti-MET drugs. On the other hand, in the vast majority of cancer types, MET acts as adjuvant metastogene (‘expedience’) (70). This notion points out that targeted therapies against MET could be effective also as a secondary approach to hamper the progression of a much wider spectrum of advanced cancers that rely on MET activation for their metastatic dissemination. Due to the relevance in lung cancer current therapeutic approach, it will be also stressed the potentiality of targeting MET in concomitance to anti-angiogenetic therapies as well as during radiotherapy.
MET activation in NSCLC
In protein studies of human lung cancer tissues, Ma et al. demonstrated MET expression in 100% of NSCLC cases studied, of which 61% stained strongly (71). When subcategorized, 67% of adenocarcinomas, 60% of carcinoids, 57% of large cell carcinomas, 57% of squamous cell carcinomas and 25% of SCLCs strongly expressed MET. When assessing for functional activity with phospho-MET staining, 44% of adenocarcinomas, 86% of large cell, 71% of squamous cell, 40% of carcinoids and 100% of SCLCs demonstrated MET phosphorylation at the Y1003 c-Cbl binding site; 33% of adenocarcinomas, 57% of large cell and 50% of SCLCs demonstrated MET autophosphorylation at the Y1230/1234/1235 site (72). In other studies of lung adenocarcinoma, 41-72% of patients showed MET expression and 25-67% showed MET overexpression in comparison to adjacent normal tissue (73,74). MET mutation was detected in 8% of lung adenocarcinoma patients and 13% of small cell lung carcinoma patients (72,75). In NSCLC, three JM domain mutations were identified: R988C was found in 1 cell line, both R988C and T1010I were found in 1 tissue, and S1058P was found in 1 tissue. Four SEMA domain missense mutations were identified: E168D was found in 1 tissue, L229F was found in 1 tissue, S323G was found in 1 tissue, and N375S was found in 3 tissues. They also identified an alternative splice variant missing exon 14 in the JM domain, which was present in 1 tissue. MET is not only mutated but is also amplified in NSCLC. Lutterbach et al. studied 9 NSCLC cell lines, of which 22% demonstrated MET amplification, up to 2.5 fold greater than normal levels (55).
MET amplification has been documented in NSCLC, especially after treatment with tyrosine kinase inhibitors. Engelman et al. demonstrated the development of MET amplification in the HCC827 NSCLC cell line after exposure to increasing concentrations of the tyrosine kinase inhibitor gefitinib (57). Cells lines that developed gefitinib resistance contained amplification of the MET-containing region 7q31.1 to 7q33.3. In an assessment of tumor tissue from 18 gefitinib-resistant NSCLC patients, 22% demonstrated MET amplification. Bean et al. also studied tissue from lung adenocarcinoma patients with gefitinib or erlotinib resistance, and found MET amplification in 21%. On the other hand, only 3% of patients who had not been treated with these drugs showed MET amplification. Hence, amplification of the MET oncogene allows tumors to potentially overcome therapeutic inhibition of growth signals.
Amplification of focal adhesion signaling molecules such as paxillin has also been demonstrated in lung cancer. Increased expression and increased copy numbers of both paxillin and MET were found in 38% of NSCLC cell lines tested; however, increased copy numbers of paxillin alone were found in 25% (76). In analysis of tissue from 66 NSCLC patients, increased copy numbers of both paxillin and MET were found in 17% of large cell carcinomas, 8% of adenocarcinomas and 13% of squamous cell carcinomas. Furthermore, 21 paxillin mutations were found among 9.4% of lung cancers studied, more frequently in large cell carcinoma (18.4%) than adenocarcinoma (8.6%), squamous cell carcinoma (6%), or small cell carcinoma (0%) (71).
Activation of the HGF-MET signalling pathway in NSCLC induces similar cellular events as those seen in SCLC. HGF-induced stimulation of adenocarcinoma induced autophosphorylation, phosphorylation of the c-Cbl binding epitope in the JM domain, and downstream phosphorylation of PI3K, PDK-1, AKT, mTOR, and S6K. The viability of MET-expressing cells was decreased by small molecule inhibitor SU11274. It also inhibited HGF-induced phosphorylation of MET and downstream signalling (66).
The role of MET in resistance to anti-EGFR molecules
Lung tumors can show de novo resistance (primary resistance) to tyrosine kinase inhibitors (TKI) therapy, even in the presence of an activating mutation in EGFR. EGFR primary resistance is mainly mediated by the occurrence of EGFR mutations or by other genomic alterations which co-occur with an EGFr mutation. However primary resistance to EGFR TKIs may also be mediated by non-mutation-based mechanisms. One of the most interesting examples involves increased expression of hepatocyte growth factor (HGF), the ligand for the MET receptor tyrosine kinase (77). HGF binding increases MET-mediated activation of the PI3K-AKT pathway, decreasing the ability of an EGFR TKI to effectively inhibit this signaling cascade. In contrast to the role of MET in acquired resistance, primary resistance owing to increased HGF activation of MET is channeled through GAB1, not ERBB3 (78). Until recently, the clinical definition of acquired resistance (secondary resistance) to EGFR TKIs in lung cancer was not uniform. To minimize reporting of false-positive and false-negative activity in clinical trials and to facilitate the identification of agents that truly overcome acquired resistance to gefitinib and erlotinib, the following clinical and molecular criteria were recently proposed to more precisely define acquired resistance to EGFR TKIs (79). The relatively simple definition should lead to a more uniform approach to investigating the problem of acquired resistance to EGFR, since several complex mechanisms have been described in inducing secondary resistance. Patients with EGFR-mutant tumors who develop acquired resistance to EGFR TKIs often develop a second-site mutation in the threonine gatekeeper residue at position 790, T790M (80). Amplification of the MET oncogene is observed in up to 20% of EGFR-mutant NSCLCs after TKI failure, independently of the T790M mutation (57,65). Cells with MET amplification seem to undergo a kinase switch and rely on MET signaling through the ERBB3 pathway to maintain activation of AKT through increased phosphorylation in the presence of EGFR TKIs. In one study, tumor cells with MET amplification were detected at a low frequency using high-throughput FISH in four patients with untreated EGFR-mutant tumors who all developed acquired resistance to gefitinib or erlotinib through MET amplification (78). By contrast, pre-existing amplification was found only rarely in tumors from patients (one of eight) who did not develop resistance by MET amplification. Collectively, these data suggest that TKI therapy may select for pre-existing cells with MET amplification suggesting that MET amplification is triggered (or selected) by EGFR kinase inhibitors such as gefitinib. These researchers also reported that in vitro studies (using H820 lung adenocarcinoma cells which harbour an EGFR mutation and MET amplification) demonstrated the MET inhibitor XL880 was more effective at inhibiting lung adenocarcinoma cell viability than EGFR inhibitors (namely erlotinib and CL-387,785). Perhaps compounds like XL880 will become an important treatment for patients with EGFR mutant lung adenocarcinomas, where MET amplification occurs and treatment resistance appears (65). Recent reports have identified Tolfenamic acid, a non-steroidal anti-inflammatory drug, as another anti-cancer molecule that can decrease MET expression. It acts by downregulating or degrading several Sp-dependent genes and proteins, especially Sp1 and Sp3, which mediate MET expression. In nude mice harboring A549 and CRL5803 human lung cancer cells, Tolfenamic acid inhibited cell survival and increased apoptosis in a dose dependent manner, ultimately causing mice to have smaller tumors (81).
Rationale and strategies to target MET in lung cancer
Therapeutic approaches that block MET signalling have being studied, and include the use of: small interference RNA, Geldanamycin, competitive HGF homologues, decoy receptors and direct MET inhibitors such as K252a, SU11274, PHA665752 and PF2341066 (for a review see Comoglio PM et al. 2008 (70) and Stella GM et al. 2010 (82). Several clinical trials are evaluating a number of these inhibitors in lung cancer, both SCLC and NSCLC. As discussed above, MET is known to play a relevant role in mediating resistance to anti EGFR therapy (83) and this observation sustains a strong rationale to combine anti EGFR and anti MET therapies. Very recently, Nakagawa et al. tested the effects of the mutant selective EGFR-TKI WZ4002 and the mutant selective Met-TKI E7050 to overcome resistance in mutant lung cancer cell lines (84). However, primary resistance to EGFR TKIs may also be mediated by non-mutation-based mechanisms. One of the most interesting examples involves increased expression of hepatocyte growth factor (HGF), the ligand for the MET receptor tyrosine kinase (85). HGF binding increases MET-mediated activation of the PI3K-AKT pathway, decreasing the ability of an EGFR TKI to effectively inhibit this signalling cascade. Primary resistance owing to increased HGF activation of MET is channelled through Gab1 Amplification of the MET oncogene is observed in up to 20% of EGFR-mutant NSCLCs after tyrosine kinase inhibitors failure (acquired or secondary resistance), independently of the T790M mutation. Cells with MET amplification seem to undergo a kinase switch and rely on MET signalling through the ERBB3 pathway to maintain activation of AKT through increased phosphorylation in the presence of EGFR TKIs. Collectively, these data suggest that TKIs therapy may select for pre-existing cells with MET amplification suggesting that MET amplification is triggered (or selected) by EGFR kinase inhibitors such as gefitinib. In vitro studies demonstrated the MET inhibitor XL880 was more effective at inhibiting lung adenocarcinoma cell viability than EGFR inhibitors. Perhaps compounds like XL880 will become an important treatment for patients with EGFR mutant lung adenocarcinomas, where MET amplification occurs and treatment resistance appears. Several molecules that can inhibit MET are now under investigation. Promising results are coming from the inhibition of the heat shot protein (hsp) 90, a chaperon for a number of several oncoproteins (86). Recent reports have identified tolfenamic acid, a non-steroidal anti-inflammatory drug, as another anti-cancer molecule that can decrease MET expression. It acts by downregulating or degrading several Sp-dependent genes and proteins, especially Sp1 and Sp3, which mediate MET expression. In nude mice harboring A549 and CRL5803 human lung cancer cells, tolfenamic acid inhibited cell survival and increased apoptosis in a dose dependent manner, ultimately causing mice to have smaller tumors (81). Another interesting molecule is certainly crizotinib, which is a dual MET and ALK inhibitor. Currently, clinical development of crizotinib is focused primarily on ALK rearranged NSCLC. However Ou et al. reported an NSCLC patient with de novo MET amplification but no ALK rearrangement who achieved a rapid and durable response to crizotinib indicating is also a bona fide MET inhibitor (87). Notably, very recently in vitro studies (88) have shown that resistance to MET inhibitors is mediated by the acquisition of a mutation in the MET activation loop. The other cause of resistance was activation of the EGFR pathway due to increased expression of transforming growth factor α. This preliminary report, of extreme interest for lung cancer, strongly suggests that resistance could be prevented by combining EGFR and MET inhibitors. MetMAb (OA-5D5) is a one-armed monoclonal antibody developed to bind to and inhibit c-MET receptor tyrosine kinase. this agent holds great promise in diseases thought to be driven by c-MET activation, as evidenced by the Phase II results in NSCLC where a benefit in overall survival was observed in patients with MET-diagnostic-positive disease (89).
On the other De Bacco et al. (64) have recently demonstrated that ionizing radiation induces overexpression and activity of the MET oncogene through the ATM-NF-κB signaling pathway; MET, in turn, promotes cell invasion and protects cells from apoptosis, thus supporting radioresistance. This correlation must be kept in consideration since radiotherapy still identifies together with chemo the standard therapeutic approach to advanced NSCLC. Interestingly, the new compound AMG-458 seems to enhance radiosensitivity in cells featuring constitutive phosphorylation of c-Met by reducing p-Akt and p-Erk levels (90). Similar results have been shown by Bhardwaj et al. (91) who demonstrated that the MET inhibitor MK-8033 radiosensitizes the high-c-Met-expressing EBC-1 and H1993 cells but not the low-c-Met-expressing cell lines A549 and H460.
Outlook
Among lung cancer genes, main relevance is currently addressed to the role of MET receptor. In most tumors the MET tyrosine kinase receptor is transcriptionally induced by hypoxia and inflammatory cytokines or pro-angiogenetic factors abundant in the reactive stroma. Hence, MET activation is a late event that aggravates the intrinsic malignant properties of already transformed cells by conveying proliferative, anti-apoptotic and pro-migratory signals (a biological situation named as ‘oncogene expedience’ (70). In a limited number of tumor cells featuring genetic lesions of MET - gene amplifications and point mutations - receptor hyperactivation in inherent in the cancer natural history and is required to maintain the transformed phenotype: these cells are dependent on MET persistent activity for their relentless proliferation (‘oncogene addiction’). The activation state of MET in the context of ‘oncogene expedience’ is dynamic because it is mainly dependent on the hectic and reversible environmental conditions typical of cancer; in this situation, MET-regulated outcomes are controlled by multiple, non-redundant signaling cross-talks. Conversely intrinsic addiction to MET is characterized by high steady-state signaling due to chronic receptor activation which is the consequence of a fixed and transmissible genetic alteration: in this setting, tumor reliance on MET continued activity appears to be governed by a small, self-sufficient group of signal transducers. Thus ‘oncogene addiction’ and ‘oncogene expedience’ should be considered as separate entities in defining therapeutic options and strategies.
With respect to NSCLC the role of MET gene activation is particularly relevant. From the perspective of its role as an expedient for tumor progression it should be noted that MET is overexpressed in response to unfavorable microenvironmental conditions such as hypoxia and ionizing radiations. On one hand it should be noted that lung cancer mainly arouses in smokers subjects who features functional obstruction which is responsible for hypoxemic conditions. Till now no studies have been focused to correlates obstruction and hypoxemic syndromes to MET overexpression in cancers. Mutations provide a mechanism for MET dysregulation in lung cancer and may be involved in addicted phenotypes. MET somatic mutations have been described with a relatively high frequency in SCLC, but they are found in NSCLC tumors also. Mutations can vary with ethnicity (92). It is well documented that amplification of the MET oncogene represents a mechanism of acquired resistance to EGFR TK inhibitors. Taken together occurrence of the EGFR T790M mutation and MET amplification stand for almost 70% of causes of acquired resistance to EGFR inhibitors in NCLC. More often the two genetic lesions arise independently in different metastases of the same tumor. This observation sustains a strong rationale for combinatorial anti-EGFR/anti-MET approach, at least in relapsed patients.
In conclusion, the receptor tyrosine kinase MET is implicated in a wide variety of human cancers, including NSCLC. Future studies will be addressed to the detection and continuous monitoring of tumor molecular biomarkers by non-invasive technologies aim to individualize anti-MET therapy, by exploiting oncogene addiction.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96.
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85.
- Trusolino L, Comoglio PM. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer 2002;2:289-300.
- Comoglio PM, Trusolino L. Cancer: the matrix is now in control. Nat Med 2005;11:1156-9.
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70.
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002;2:442-54.
- Comoglio PM, Boccaccio C. Scatter factors and invasive growth. Semin Cancer Biol 2001;11:153-65.
- Cross M, Dexter TM. Growth factors in development, transformation, and tumorigenesis. Cell 1991;64:271-80.
- Nakamura T, Teramoto H, Ichihara A. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc Natl Acad Sci USA 1986;83:6489-93.
- Stoker M, Gherardi E, Perryman M, et al. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature 1987;327:239-42.
- Naldini L, Weidner KM, Vigna E, et al. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J 1991;10:2867-78.
- Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol 1997;137:1403-19.
- Schmidt L, Duh FM, Chen F, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet 1997;16:68-73.
- Uehara Y, Minowa O, Mori C, et al. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 1995;373:702-5.
- Bladt F, Riethmacher D, Isenmann S, et al. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 1995;376:768-71.
- Nagafuchi A. Molecular architecture of adherens junctions. Curr Opin Cell Biol 2001;13:600-3.
- Tannapfel A, Wittekind C, Tahara E. Effect of hepatocyte growth factor (HGF)/scatter factor (SF) on cell adhesion in gastric cancer. Z Gastroenterol 1994;32:91-3.
- Miura H, Nishimura K, Tsujimura A, et al. Effects of hepatocyte growth factor on E-cadherin-mediated cell-cell adhesion in DU145 prostate cancer cells. Urology 2001;58:1064-9.
- Balkovetz DF, Pollack AL, Mostov KE. Hepatocyte growth factor alters the polarity of Madin-Darby canine kidney cell monolayers. J Biol Chem 1997;272:3471-7.
- Balkovetz DF. Evidence that hepatocyte growth factor abrogates contact inhibition of mitosis in Madin-Darby canine kidney cell monolayers. Life Sci 1999;64:1393-401.
- Davies G, Jiang WG, Mason MD. Matrilysin mediates extracellular cleavage of E-cadherin from prostate cancer cells: a key mechanism in hepatocyte growth factor/scatter factor-induced cell-cell dissociation and in vitro invasion. Clin Cancer Res 2001;7:3289-97.
- Shibamoto S, Hayakawa M, Takeuchi K, et al. Tyrosine phosphorylation of beta-catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells. Cell Adhes Commun 1994;1:295-305.
- Trusolino L, Serini G, Cecchini G, et al. Growth factor-dependent activation of alphavbeta3 integrin in normal epithelial cells: implications for tumor invasion. J Cell Biol 1998;142:1145-56.
- Trusolino L, Cavassa S, Angelini P, et al. HGF/scatter factor selectively promotes cell invasion by increasing integrin avidity. FASEB J 2000;14:1629-40.
- Brooks PC, Strömblad S, Sanders LC, et al. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell 1996;85:683-93.
- Nabeshima K, Inoue T, Shimao Y, et al. Front-cell-specific expression of membrane-type 1 matrix metalloproteinase and gelatinase A during cohort migration of colon carcinoma cells induced by hepatocyte growth factor/scatter factor. Cancer Res 2000;60:3364-9.
- Monvoisin A, Bisson C, Si-Tayeb K, et al. Involvement of matrix metalloproteinase type-3 in hepatocyte growth factor-induced invasion of human hepatocellular carcinoma cells. Int J Cancer 2002;97:157-62.
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000;407:249-57.
- Bottaro DP, Rubin JS, Faletto DL, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 1991;251:802-4.
- Maina F, Casagranda F, Audero E, et al. Uncoupling of Grb2 from the Met receptor in vivo reveals complex roles in muscle development. Cell 1996;87:531-42.
- Bardelli A, Longati P, Gramaglia D, et al. Uncoupling signal transducers from oncogenic MET mutants abrogates cell transformation and inhibits invasive growth. Proc Natl Acad Sci USA 1998;95:14379-83.
- Cooper CS, Park M, Blair DG, et al. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 1984;311:29-33.
- Ponzetto C, Bardelli A, Zhen Z, et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 1994;77:261-71.
- Boccaccio C, Andò M, Tamagnone L, et al. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature 1998;391:285-8.
- Pelicci G, Lanfrancone L, Salcini AE, et al. Constitutive phosphorylation of Shc proteins in human tumors. Oncogene 1995;11:899-907.
- Weidner KM, Di Cesare S, Sachs M, et al. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature 1996;384:173-6.
- Maroun CR, Naujokas MA, Holgado-Madruga M, et al. The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol Cell Biol 2000;20:8513-25.
- Royal I, Park M. Hepatocyte growth factor-induced scatter of Madin-Darby canine kidney cells requires phosphatidylinositol 3-kinase. J Biol Chem 1995;270:27780-7.
- Ponzetto C, Zhen Z, Audero E, et al. Specific uncoupling of GRB2 from the Met receptor. Differential effects on transformation and motility. J Biol Chem 1996;271:14119-23.
- Gual P, Giordano S, Williams TA, et al. Sustained recruitment of phospholipase C-gamma to Gab1 is required for HGF-induced branching tubulogenesis. Oncogene 2000;19:1509-18.
- Zeng Q, Chen S, You Z, et al. Hepatocyte growth factor inhibits anoikis in head and neck squamous cell carcinoma cells by activation of ERK and Akt signaling independent of NF_B. J Biol Chem 2002;277:25203-8.
- Boccaccio C, Ando’ M, Comoglio PM. A differentiation switch for genetically modified hepatocytes. FASEB J 2002;16:120-2.
- Karihaloo A, O’Rourke DA, Nickel C, et al. Differential MAPK pathways utilized for HGF- and EGF-dependent renal epithelial morphogenesis. J Biol Chem 2001;276:9166-73.
- Bertotti A, Comoglio PM. Tyrosine kinase signal specificity: lessons from the HGF receptor. Trends Biochem Sci 2003;28:527-33.
- Trusolino L, Bertotti A, Comoglio PM. A signaling adapter function for alpha6beta4 integrin in the control of HGF-dependent invasive growth. Cell 2001;107:643-54.
- Giordano S, Corso S, Conrotto P, et al. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol 2002;4:720-4.
- Orian-Rousseau V, Chen L, Sleeman JP, et al. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev 2002;16:3074-86.
- Wang R. Ferrell LD, Fauozi S, et al. Activation of the MET receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol 2001;153:1023-34.
- Lokker NA, Mark MR, Luis EA, et al. Structure-function analysis of hepatocyte growth factor: identification of variants that lack mitogenic activity yet retain high affinity receptor binding. EMBO J 1992;11:2503-10.
- Hartmann G, Naldini L, Weidner KM, et al. A functional domain in the heavy chain of scatter factor/hepatocyte growth factor binds the c-Met receptor and induces cell dissociation but not mitogenesis. Proc Natl Acad Sci USA 1992;89:11574-8.
- Waltz SE, McDowell SA, Muraoka RS, et al. Functional characterization of domains contained in hepatocyte growth factor-like protein. J Biol Chem 1997;272:30526-37.
- Matsumoto K, Kataoka H, Date K, et al. Cooperative interaction between - and -chains of hepatocyte growth factor on c-Met receptor confers ligand-induced receptor tyrosine phosphorylation and multiple biological responses. J Biol Chem 1998;273:22913-20.
- Di Renzo MF, Olivero M, Giacomini A, et al. Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin Cancer Res 1995;1:147-54.
- Smolen GA, Sordella R, Muir B, et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci USA 2006;103:2316-21.
- Lutterbach B, Zeng Q, Davis LJ, et al. Lung cancer cell lines harboring MET gene amplification are dependent on Met for growth and survival. Cancer Res 2007;67:2081-8.
- Di Renzo MF, Olivero M, Martone T, et al. Somatic mutations of the MET oncogene are selected during metastatic spread of human HNSC carcinomas. Oncogene 2000;19:1547-55.
- Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007;316:1039-43.
- Schmidt L, Duh FM, Chen F, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET protooncogene in papillary renal carcinomas. Nat Genet 1997;16:68-73.
- Park WS, Dong SM, Kim SY, et al. Somatic mutations in the kinase domain of the Met/hepatocyte growth factor receptor gene in childhood hepatocellular carcinomas. Cancer Res 1999;59:307-10.
- Lee JH, Han SU, Cho H, et al. A novel germ line juxtamembrane Met mutation in human gastric cancer. Oncogene 2000;19:4947-53.
- Ma PC, Kijima T, Maulik G, et al. c-MET mutational analysis in small cell lung cancer: novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res 2003;63:6272-81.
- Lorenzato A, Olivero M, Patanè S, et al. Novel somatic mutations of the MET oncogene in human carcinoma metastases activating cell motility and invasion. Cancer Res 2002;62:7025-30.
- Pennacchietti S, Michieli P, Galluzzo M, et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 2003;3:347-61.
- De Bacco F, Luraghi P, Medico E, et al. Induction of MET by ionizing radiation and its role in radioresistance and invasive growth of cancer. J Natl Cancer Inst 2011;103:645-61.
- Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA 2007;104:20932-7.
- Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol 2010;11:834-48.
- Siegfried JM, Weissfeld LA, Singh-Kaw P, et al. Association of immunoreactive hepatocyte growth factor with poor survival in resectable non-small cell lung cancer. Cancer Res 1997;57:433-9.
- Boccaccio C, Sabatino G, Medico E, et al. The MET oncogene drives a genetic programme linking cancer to haemostasis. Nature 2005;434:396-400.
- Michieli P, Mazzone M, Basilico C, et al. Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell 2004;6:61-73.
- Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov 2008;7:504-16.
- Ma PC, Jagadeeswaran R, Jagadeesh S, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res 2005;65:1479-88.
- Ichimura E, Maeshima A, Nakajima T, et al. Expression of c-met/HGF receptor in human non-small cell lung carcinomas in vitro and in vivo and its prognostic significance. Jpn J Cancer Res 1996;87:1063-9.
- Olivero M, Rizzo M, Madeddu R, et al. Overexpression and activation of hepatocyte growth factor/scatter factor in human non-small-cell lung carcinomas. Br J Cancer 1996;74:1862-8.
- Tsao MS, Liu N, Chen JR, et al. Differential expression of Met/hepatocyte growth factor receptor in subtypes of non-small cell lung cancers. Lung Cancer 1998;20:1-16.
- Ma PC, Salgia R. Novel targets for therapeutic agents in small cell lung cancer. J Natl Compr Canc Netw 2004;2:165-72.
- Jagadeeswaran R, Surawska H, Krishnaswamy S, et al. Paxillin is a target for somatic mutations in lung cancer: implications for cell growth and invasion. Cancer Res 2008;68:132-42.
- Yano S, Wang W, Li Q, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res 2008;68:9479-87.
- Turke AB, Zhejnullahu K, Wu YL, et al. Preexistence and Clonal Selection of MET Amplification in EGFR Mutant NSCLC. Cancer Cell 2010;17:77-88.
- Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol 2010;28:357-60.
- Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73.
- Colon J, Basha MR, Madero-Visbal R, et al. Tolfenamic acid decreases c-Met expression through Sp proteins degradation and inhibits lung cancer cells growth and tumor formation in orthotopic mice. Invest New Drugs 2011;29:41-51.
- Stella GM, Benvenuti S, Comoglio PM. Targeting the MET oncogene in cancer and metastases. Expert Opin Investig Drugs 2010;19:1381-94.
- Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer 2010;10:760-74.
- Nakagawa T, Takeuchi S, Yamada T, et al. Combined therapy with mutant-selective EGFR inhibitor and Met kinase inhibitor for overcoming erlotinib resistance in EGFR mutant lung cancer. Mol Cancer Ther 2012. [Epub ahead of print].
- Yano S, Wang W, Li Q, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res 2008;68:9479-87.
- Giaccone G, Rajan A. Met amplification and HSP90 inhibitors. Cell Cycle News & Views 2009;8:2681-4.
- Ou SH, Kwak EL, Siwak-Tapp C, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol 2011;6:942-6.
- Qi J, McTigue MA, Rogers A, et al. Multiple mutations and bypass mechanisms can contribute to development of acquired resistance to MET inhibitors. Cancer Res 2011;71:1081-91.
- Surati M, Patel P, Peterson A, et al. Role of MetMAb (OA-5D5) in c-MET active lung malignancies. Expert Opin Biol Ther 2011;11:1655-62.
- Li B, Torossian A, Sun Y, et al. Higher Levels of c-Met Expression and Phosphorylation Identify Cell Lines With Increased Sensitivity to AMG-458, a Novel Selective c-Met Inhibitor With Radiosensitizing Effects. Int J Radiat Oncol Biol Phys 2012. [Epub ahead of print].
- Bhardwaj V, Zhan Y, Cortez MA, et al. C-Met Inhibitor MK-8003 Radiosensitizes c-Met-Expressing Non-Small-Cell Lung Cancer Cells With Radiation-Induced c-Met-Expression. J Thorac Oncol 2012;7:1211-7.
- Lawrence RE, Salgia R. MET molecular mechanisms and therapies in lung cancer. Cell Adh Migr 2010;4:146-52.


