Triaging early-stage lung cancer patients into non-surgical pathways: who, when, and what?
Background
Fundamental questions
Early-stage lung cancer, defined for the purposes of this article as <5 cm in maximal diameter and node negative (cT2aN0M0 or earlier, and T3N0M0 if due to parietal, mediastinal or pericardial pleura involvement), accounts for approximately 15-20% of all newly diagnosed lung cancer cases (1). Surgery has always been considered the standard treatment for patients with early-stage lung cancer. When surgery cannot be performed, due to medical reasons such as poor pulmonary function, poor performance status, medical comorbidities, or patient refusal, alternatives to surgery have to be sought. The immediate question that arises in this scenario is: ‘what are non-surgical alternative treatment modalities and how do the outcomes compare to surgery?’ With wider adoption and improving results from non-surgical treatment options, another question has recently arisen: ‘will any alternative modality threaten the position of surgery as the standard of care treatment for early-stage non-small cell lung cancer (NSCLC)?’
Before these questions, was a more fundamental one: ‘do patients with early-stage inoperable lung cancer need upfront treatment, or can they be managed with a watchful waiting approach similar to what is used in early-stage prostate cancer in medically unfit or elderly patients?’ Jeppesen et al. (2) retrospectively compared stereotactic body radiation therapy (SBRT) to no treatment in patients with medically inoperable T1-2N0 NSCLC. Respectively, the mean age was 73 vs. 78 years; the mean tumor size was 3.2 vs. 3.7 cm; the median overall survival (OS) from the date of diagnosis was 40 vs. 9.9 months; the 5-year OS was 37% and 6%. Among the patients in the untreated group, 77% died from lung cancer vs. 39% in the SBRT treatment group. Multivariate analysis showed that treatment with SBRT, performance status and age had a significant influence on survival. While there is obvious selection bias in a retrospective study such as this, treatment whenever possible is clearly beneficial. This brings us back to the initial question about the optimal treatment for patients with early-stage inoperable NSCLC.
Options for non-surgical treatment of early-stage NSCLC
Radiofrequency ablation (RFA)
RFA is a minimally invasive technique employed by interventional radiologists. RFA directly damages tumors thermally through electromagnetic energy deposition. A radiofrequency generator produces an alternating current that moves from an active electrode inserted into the tumor to dispersive electrodes placed on the surface of the patient. This high-frequency electrical current heats and coagulates tissue, generating intratumoral temperatures above 60 °C, resulting in cell death through protein denaturation and coagulation necrosis (3).
Pros and cons
The main advantage of RFA is it can be performed in an outpatient setting under local anesthesia. Lung tumors are ideal RFA targets as the damage to surrounding tissues is mitigated due to the presence of air, providing an insulating effect by allowing rapid heat dissipation. A major limitation of RFA is the inability to deliver treatment to targets in close proximity to blood vessels larger than 3 mm. This proximity causes loss of energy and heat through convection into the circulatory system, resulting in less energy delivered to the target, the so-called “heat sink effect” (4). Tumor size is another limiting factor. Local control (LC) is reduced in targets greater than 3 cm. As target volume increases, the periphery receives less energy, resulting in potentially non-ablative temperatures and reduced LC (5). Also, RFA is contraindicated in tumors located within 1 cm of the esophagus, trachea, great vessels or mainstem bronchi. The most common complications of RFA are pneumothorax, hemoptysis, bronchopleural fistula, and rib fracture.
Supportive clinical evidence
The majority of data regarding the efficacy of RFA in the treatment of early-stage primary lung cancer are retrospective; many of these reports combined data from treatment of both primary lung cancer and metastatic tumors to the lung. Looking specifically at early-stage primary NSCLC, Beland et al. (6) evaluated 79 patients with 79 primary lung tumors treated with RFA. The median tumor size was 2.5 cm (range: 1-5.5 cm), median follow-up was 16 months. LC was 57% with a median disease free survival (DFS) of 23 months. Lanuti et al. (7) reviewed 31 patients with 34 peripheral primary lung tumors (29 T1N0 and 5 T2N0) with a mean tumor size of 2 cm. After median follow-up of 17 months, LC was 68.5%, the 1-, 2- and 3-year OSs were 85%, 78% and 47%, respectively; DFS was 57% at 2 years and 39% at 3 years; and median DFS was 25.5 months. Reported complications were pneumothorax in 13% of patients, pneumonia in 16% and pleural effusion in 21%.
Outcomes in the treatment of early-stage NSCLC using RFA appear to be very dependent on the skill and experience of those performing the procedure, but LC rates are generally inferior compared to SBRT. In a literature review, Bi et al. (8) found that for stage I NSCLC the local tumor control rate at 3-year was 55% for RFA and 88% for SBRT. However, there was no difference between the two modalities in terms of OS, however. More prospective and randomized evidence is needed to determine the efficacy and role of RFA in the treatment of early-stage NSCLC. American College of Surgeons Oncology Group Z4033, a phase II trial examining outcomes of high risk patients with early-stage NSCLC treated with RFA, is currently closed to accrual but data from this study have not yet been reported.
Microwave ablation (MWA)
MWA is a relatively new technique in the treatment of lung cancer and similar to RFA, it is typically delivered percutaneously under CT-guidance. Unlike RFA, tumor cell killing is a result of electromagnetic waves that produce excitation and oscillation of water molecules within the tissue surrounding the probe (9).
Pros and cons
Potential advantages of MWA compared to RFA include enhanced thermocoagulation of tumor cells as a result of improved energy deposition in aerated lung, increased temperatures within the tumor in a shorter amount of time and a larger ablative area. MWA may allow for improved treatment of central lesions compared to RFA because of minimal heat sink effect associated with surrounding vasculature. The most common complications of MWA are similar to those of RFA, including risk of pneumothorax, post-procedure pain and hemoptysis.
Supportive clinical evidence
Unfortunately, despite the potential advantages, the limited data about MWA have not shown superior outcomes compared to RFA. Wolf et al. (10) published retrospective results of percutaneous CT-guided MWA in 82 primary and metastatic lung lesions in 50 patients. With a median follow-up of 10 months, 1-year LC was 67%; 26% of patients had residual disease at the ablation site, which is similar to RFA; OS at 1, 2 and 3 years was 83%, 73% and 61% respectively.
Percutaneous cryoablation therapy (PCT)
PCT is a thermoablative technique that utilizes cold instead of heat, as used in RFA and MWA. A gas, usually Argon, rapidly decreases in temperature (as low as −150 °C) after transitioning from a liquid to a gaseous state. A probe inserted into a patient can deliver a freeze area of 2-3 cm diameter. A freeze cycle is followed by a thaw cycle, where Helium gas is administered to raise the temperature to around 40 °C. The freeze and thaw cycles are alternated. Rapid freezing causes direct damage to tumors by forming intracellular and extracellular ice crystals, which disrupt the cell membrane and intracellular processes. Hypoxic cell death occurs indirectly from vasoconstriction and occlusion of adjacent vasculature. The diameter and number of probes, and number of freeze/thaw cycles can be varied depending on the size and location of the target (11).
Pros and cons
Similar to RFA, PCT is recommended for tumors less than 3 cm. Larger tumors cause difficult geometry and difficulty in probe placement, resulting in lower control rates. PCT also suffers from the heat/cold sink effect like RFA. In contrast to RFA, PCT can be utilized safely for central tumors due to the relative resistance of collagenous airway structures (12). The most significant complications of PCT, similar to RFA and MWA, are pneumothorax, hemorrhage, fistula formation and bronchospasm. Cryoablation therapy may result in less pain along the pleura and chest wall compared to RFA and MWA.
Supportive clinical evidence
The data evaluating PCT in the management of early-stage lung cancer are very limited but generally promising. Yamauchi et al. (13) retrospectively reviewed the results of 34 tumors in 22 patients with stage I NSCLC with a mean tumor diameter of 1.4 cm. After a median follow-up of 23 months, only 1 tumor progressed locally (3%), the 2- and 3-year DFS were 78% and 67%, respectively; median OS was 68 months.
Photodynamic therapy (PDT)
PDT is a technique which has been used more frequently in the treatment of thoracic malignancies over the last several years, but is still not available at many institutions. In PDT, a photosensitizing agent is delivered systemically, followed by direct excitation of the agent by a wavelength of light that correlates to the absorption band of the infused drug. This reaction results in the production of oxygen free radicals and singlet oxygen, highly reactive states of oxygen. Cell death occurs due to direct injury via both apoptosis and cell necrosis. Indirect damage occurs as a result of damage to the tumor vasculature and from local inflammatory response with associated antitumor cytokines (14-17).
Pros and cons
Complications of PDT include hemoptysis, pulmonary toxicity and skin burns related to systemic delivery of the drug and exposure to UV light (12). PDT generally has a role in the treatment of centrally located, small (<1 cm) early-stage and non-invasive tumors. Small tumors that are minimally invasive and without extension beyond the bronchial wall are the optimal targets for PDT because they allow light to penetrate the target tissue and activate the photosensitizing agent (14).
Supportive clinical evidence
Furuse et al. (18) published a phase II study reporting on the use of the photosensitizing agent porfimer sodium (Photofrin II) in the treatment of 49 patients with 59 early-stage, centrally located squamous cell carcinoma tumors. Overall 85% of tumors showed a complete response at a median follow-up of 14 months. Tumors <5 mm had a 100% complete response rate and tumors >20 mm had a 38% complete response rate. Kato et al., (19) also showed the size of the tumor was a strong predictor of outcome in a study in which 204 patients with 264 central, early-stage NSCLC tumors were treated with PDT. The complete response rate was 95% in tumors <5 mm, 94% in tumors 5-9 mm and 44% in tumors >20 mm.
External beam radiation therapy (EBRT)
EBRT has been the alternative treatment of choice for early-stage NSCLC. Traditional fractionation schemes using 1.8 to 2 Gy per fraction per day have had local failure rates exceeding 50% historically, leading to long-term survival rates of only 15-30% (20-22). It was assumed that doses in excess of 85 Gy were likely necessary to achieve a LC of more than 50% when using standard fractions. However, this would have led to excessively long overall treatment times. Given the age, comorbidities and performance status of the typical inoperable lung cancer patient, this is not a small problem. Also, due to accelerated repopulation, there is a 1.6% per day loss in survival with prolongation of treatment beyond 6 weeks (23-25).
SBRT, also known as stereotactic ablative body radiotherapy (SABR), is characterized by a high radiation dose (tumor biologically effective doses generally ≥100 Gy), and highly conformal dose distribution with rapid dose fall-off. It entails daily image guidance for reduction in overall treatment volume, resulting in decreased dose to surrounding normal tissues and therefore decreased toxicity. Reproducible rigid immobilization is necessary, with precise measurement and minimization of set up error. Strategies should also be applied to control the respiratory motion of tumor and normal tissue during treatment planning and delivery of each fraction (26). Treatments are typically delivered in three to five fractions of 10-20 Gy each over a 1-2-week period.
RTOG 0915 compared 48 Gy in four fractions to 34 Gy in a single fraction and was presented as an abstract at the 2013 ASTRO conference (27). The median follow-up was 20.6 months and the trial met prespecified criteria for adverse events and tumor control. The treatment regimen of a single 34 Gy fraction has been selected as the experimental arm for a future phase III RTOG trial. The effectiveness of SBRT is attributed primarily to the diminished role of accelerated repopulation due to reduction in overall treatment time, and to its ability to deliver an increased biological effective dose (BED) via large fraction sizes compared to traditional fractionation.
Cons
Common major toxicities with SBRT are pneumonitis, chest wall/skin injury or rib fracture, pleural effusion, brachial plexopathy, bronchial stenosis, bronchial necrosis with potential for fatal hemoptysis, and esophagitis with potential for stricture, perforation or fistula formation.
Supportive clinical evidence
Data in support of SBRT are predominantly retrospective in nature. High LC rates of 80-97% are consistent among these studies (1). However some prospective data are available. Timmerman et al. (28) reported RTOG 0236, a prospective phase II trial which included 55 medically inoperable patients with peripheral tumors <5 cm treated with SBRT, in 2010. The median follow-up was 34.4 months. The 3-year LC, DFS and OS were 97.6%, 48.3% and 55.8%, respectively. The rate of disseminated recurrence at 3 years was 22.1%, with only two regional failures (3.6%). These findings were very encouraging and led many to conclude that SBRT should be the standard primary local therapy for inoperable early-stage NSCLC. The recently updated 5-year data from RTOG 0236 showed a total of four primary tumor failures (7%), but an overall local recurrence rate of 20%, primarily due to intralobar failures. The 5-year locoregional and distant failure rates were 38% and 31%, respectively. Fifteen patients experienced grade 3 toxicity, and two patients experienced grade 4 toxicity, but there were no deaths attributed to radiation therapy (29).
The treatment of centrally located tumors, defined as within 2 cm of the proximal bronchial tree, with SBRT has been associated with increased major complications in some trials and is considered somewhat controversial. Timmerman et al. (30) reported on 70 patients with T1 or T2 tumors ≤7 cm with biopsy-proven NSCLC treated with 60-66 Gy in three fractions over 1-2 weeks. With median follow-up of 17.5 months, LC at 2 years was 95%. However, 14 patients had grade 3-5 toxicity with six deaths due to treatment related complications. The treatment related deaths were attributed to pericardial effusion in one patient, hemoptysis in another patient and bacterial pneumonia in four other patients. The median time to onset of toxicity was 10.5 months. At 2 years, freedom from severe toxicity was 83% for peripheral tumors and 54% for central tumors. Some have advocated for a more conservative treatment approach of accelerated hypofractionation for central tumors. One such regimen uses eight fractions of 7.5 Gy per day which may approximate the effects of SBRT by still delivering a high biological equivalent dose (31).
However, if dose constraints [such as those published in Task Group 101 by American Association of Physics in Medicine (32)] are respected, SBRT can be effectively and safely utilized for central tumors with outcomes similar to those of peripheral tumors. Chang et al. (33) published a prospective trial looking at 100 patients with biopsy-proven central T1-T2N0 NSCLC or isolated parenchymal recurrence of NSCLC treated with SBRT to 50 Gy in four fractions or with accelerated hypofractionation to 70 Gy in ten fractions if dose constraints could not be met. Eighty-two patients received 50 Gy in four fractions and 18 patients received 70 Gy in ten fractions. The median follow-up time was 30.6 months; the 3-year OS was 70.5%; the 3-year local, regional and distant control rates were 96.5%, 87.9% and 77.2%, respectively. The most common toxicities were chest-wall pain (18% grade 1 and 13% grade 2) and radiation pneumonitis (11% grade 2 and 1% grade 3). There were no treatment related deaths. The optimal dose and fractionation scheme for centrally located tumors is not known, but RTOG 0813 is a Phase I/II trial looking at outcomes of various fractionation schemes for the treatment of these tumors. The trial has closed and results are pending.
Standard fractionation vs. SBRT
Improvements in imaging and treatment techniques have increased the accuracy and therapeutic ratio of all forms of EBRT, and many have wondered if modern EBRT using standard fractions can offer outcomes comparable to SBRT. Nyman et al. (34) presented a prospective randomized trial at the European Society for Therapeutic Radiology and Oncology annual meeting in 2014 comparing 2 Gy ×35 fractions of conventionally fractionated EBRT to 22 Gy ×3 fractions of SBRT. The trial enrolled 102 patients with T1-2N0 cancers at nine Scandinavian centers from 2007 to 2011. Patients in the conventionally fractionated arm were older (median age 75.3 vs. 72.7 years), but there were more T2 tumors in the SBRT arm (47% vs. 25%). The trial showed no difference between the two modalities in terms of LC or OS. However, there was significantly more treatment related toxicity in the conventionally fractionated arm due to pneumonitis (34% vs. 16%) and esophagitis (32% vs. 9%), but overall severity of toxicity was low with grade ≥3 toxicity occurring in 16% of patients in the conventionally fractionated arm and in 18% of patients in the SBRT arm. The tumor control and survival outcomes may be similar between SBRT and conventional fractionation using modern techniques, but the therapeutic ratio and overall convenience of SBRT are clearly superior. In patients who are not candidates for SBRT due to pulmonary dysfunction, many support a treatment approach using accelerated hypofractionation which can be safely delivered to these patients using a number of different fractionation schemes (35).
Table 1 compares the approximate 1-year LC rates, 2-year OS rates, common toxicities and relative contraindications for the treatment modalities discussed above. Lobectomy and sublobar resection have been added for comparison. Wedge resection was not included because of level I evidence showing clearly inferior outcomes compared to lobectomy (36). It is important to note that when comparing survival outcomes, surgical series typically include much fitter patients for whom better survival is expected than the less fit, medically inoperable patients typically receiving alternative therapies.

Full table
The future: SBRT vs. resection for surgically fit patients
Although lung cancer as a diagnosis is decreasing overall, more patients are being diagnosed at an earlier stage with improved diagnostic imaging techniques. Now that lung cancer screening is becoming standard practice, the treatment of these patients will become even more important in the future. It is estimated that screening will increase the number of lung cancer diagnoses by about 11,000 per year and that the percentage of lung cancers diagnosed at an early-stage will increase from 15% currently to 33% (37). Surgery has always been the treatment of choice for these patients, but emerging data are putting these beliefs into question. Surgery is the only treatment in early-stage NSCLC which is both diagnostic and therapeutic. Formal lymph node assessment at the time of surgery often identifies patients with more advanced disease than originally found on pre-treatment work-up. This information is then used to guide post-treatment decisions in regard to adjuvant chemotherapy and radiation therapy. Wilson et al. (38) found the rate of nodal upstaging after surgery to be 11%.
SBRT has been considered the primary challenger to surgery given the excellent results in terms of LC. Even though the locoregional failure rate for SBRT may be higher than once believed, there are emerging data that cast some doubt on the belief surgery should be the standard treatment approach for all patients with early-stage NSCLC. Shirvani et al. (39) published a SEER-Medicare propensity matched analysis comparing SBRT to observation, lobectomy, sublobar resection and conventional radiation therapy. There was no significant difference in OS for SBRT compared to lobectomy or sublobar resection. Grills et al. (40) examined 124 patients with Stage I NSCLC who received either wedge resection or SBRT. Although the SBRT patients were significantly older and had higher comorbidity index scores, the patients receiving SBRT had better LC and there was no difference in the rate of distant metastases or in cause-specific survival (CSS). OS was better in the wedge resection group, but again the SBRT patients were more unfit.
Conversely, when patients are at very high risk, especially those with severe chronic obstructive pulmonary disease (COPD) (predicted postoperative FEV1 <40%), the higher mortality risk associated with surgical resection needs to be taken into account. On average these patients have a 10% 30-day mortality risk after surgical resection compared to 0% after SBRT (41).
It has been very difficult to obtain level I evidence in the comparison of SBRT to surgery. Verstegen et al. (42) published a propensity score-matched analysis comparing SBRT to lobectomy via video-assisted thoracoscopic surgery (VATS) for stage I-II NSCLC. The matched cohort consisted of 64 SBRT and 64 VATS lobectomy patients with a median follow-up of 30 and 16 months, respectively. Locoregional control for SBRT was significantly better at 1 and 3 years (96.8 and 93.3% vs. 86.9% and 82.6%, respectively). Distant recurrences and OS were not significantly different. Zheng et al. (43) published a meta-analysis of 40 SBRT studies (4,850 patients) and 23 surgical studies (7,071 patients) published between 2000 and 2012. Patients typically treated with SBRT differ substantially from patients typically treated with surgery in age and operability. After adjustment for these differences, they found OS and DFS did not differ significantly between patients with stage I operable NSCLC treated with either SBRT or surgery.
In terms of prospective data, three Phase III randomized controlled trials (ACOSOG Z4099/RTOG 1021, ROSEL and STARS trials) recently closed early due to poor accrual. Since both arms of these studies are available off trial, this is understandable. However, Chang et al. (44) recently reported the pooled analysis from the limited patients enrolled on the ROSEL and STARS trials. The 58 total patients enrolled (31 to SBRT and 27 to surgery) were followed for a median of 40.2 months in the SBRT group and 35.4 months in the surgery group. Six patients in the surgery group died compared to one in the SBRT group. OS at 3 years was significantly better in the SBRT group compared to the surgery group (95% vs. 79%). There was one regional recurrence in the surgery group compared to four in the SBRT group. There were two distant recurrences in the surgery group compared to one in the SBRT group.
Hopefully, these results will encourage further randomized studies comparing SBRT and surgery. Currently, there are at least two studies ongoing to compare SBRT and lobectomy. RTOG foundation study 3502 is enrolling patients in China. Veterans Affairs Lung cancer surgery Or stereotactic Radiotherapy (VALOR) trial has just secured funding and finalized the protocol with patient enrollment starting in early 2016. With more robust level I data, SBRT could unseat surgery as the clear standard of care for operable early-stage NSCLC patients. Given the relative convenience and superior side effect profile (morbidity and, in some cases, mortality), data showing comparable control and survival outcomes should make SBRT the preferred treatment.
SBRT vs. other non-surgical options for non-surgical patients
For inoperable early-stage NSCLC patients, should SBRT be considered the standard treatment, or are other treatment modalities such as RFA, MWA, PCT or PDT reasonable options as well? RFA is the best studied of these techniques. Zemlyak et al. (45) compared RFA, sublobar resection and PCT retrospectively in 64 patients with inoperable stage I NSCLC. However, only nine of these patients were treated with PCT. Median follow-up was 33 months. 3-year CSS was 90.6%, 87.5% and 87.5% for sublobar resection, RFA and PCT. OS was 60.8%, 87.1% and 77% respectively. There was a statistically nonsignificant trend towards worse local and regional/distant recurrence in the RFA group. Kwan et al. (46) used the NCI SEER-Medicare database to examine outcomes of patients with early-stage NSCLC after thermal ablation compared to sublobar resection. The patients in the RFA group were older, had higher comorbidity index scores and were more likely to have COPD. Comparing these unmatched groups, there was significantly better OS and CSS in the sublobar resection group. However, after propensity score matching, OS and CSS were not significantly different.
Bilal et al. (47) performed a meta-analysis comparing RFA to SBRT. A total of 16 studies (nine studies for RFA and seven studies for SBRT) were involved. They found that the OS at 1 year (68.2–95% vs. 81–85.7%) and 3 years (36–87.5% vs. 42.7–56%) was similar between patients treated with RFA and SBRT, while 5-year OS was higher in SBRT (47%) than RFA (20.1–27%). Local progression rates were lower in patients treated with SBRT (3.5–14.5% vs. 23.7–43%). Local failure rates in RFA appear to be higher than those with SBRT or surgery. However, it is unclear how much these higher failure rates impact survival, if at all. One area where RFA has carved out a clear niche is in salvaging SBRT failures. Kodama et al. (48) treated 44 consecutive patients with recurrent NSCLC after treatment with SBRT. During the 29 months follow-up period, the 1-, 3- and 5-year OS rates were 98%, 73% and 56%. The 1- and 3-year recurrence free survival rates were 77% and 41%, respectively.
The data for MWA, PCT and PDT are too limited to draw significant conclusions regarding their efficacy versus other techniques, but retrospective data are promising and suggest that these treatment modalities should be further investigated. PCT in particular appears to have very high LC rates similar to SBRT. A cost-effectiveness analysis published by Sher et al. (49) comparing conventional RT, SBRT and RFA for medically inoperable Stage I NSCLC found that SBRT may be the most cost-effective alternative treatment for medically inoperable Stage I NSCLC.
Summary
SBRT can be performed safely with excellent outcomes on centrally located tumors as long as dose constraints are respected. If the dose constraints cannot be met and SBRT is contraindicated, accelerated hypofractionation should be utilized. The results of RTOG 0813 will help determine the optimal dose and fractionation for central tumors. Compared to SBRT, conventional fractionation using modern techniques appears to be equivalent in terms of outcomes but worse in terms of toxicity and convenience due to much longer overall treatment times. If available, cryotherapy and PDT (and to a lesser extent RFA and MWA) can also be considered for central tumors when SBRT is contraindicated but need further study.
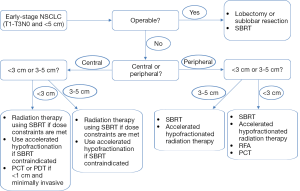
In conclusion, the treatment options for patients with early-stage NSCLC have evolved significantly over the past decade with many new and exciting treatments now available. Many of these treatments are still relatively new and have insufficient clinical trials evidence; hence, their optimal role has not yet been determined. Treatment recommendations should be individualized to the patient, based primarily on the size and location of the tumor, the patient’s age, comorbidities and performance status, and the strength of the available evidence. Based on these factors, Figure 1 presents an algorithm for determining the preferred primary treatment in early-stage NSCLC patients. As early data mature and future directions are determined, much of this will surely change.
Acknowledgements
The authors wish to generously thank Angela Wortham, MD, Roger Wortham, MD and Raymond Osarogiagbon, MD who assisted in the proof-reading of the manuscript.
Footnote
Conflicts of Interest: Rameses Sroufe—No conflicts. Feng-Ming (Spring) Kong—Varian research grant and speaker honorarium, NIH/NCI awards.
References
- Jones GC, Kehrer JD, Kahn J, et al. Primary Treatment Options for High-Risk/Medically Inoperable Early Stage NSCLC Patients. Clin Lung Cancer 2015. [Epub ahead of print]. [PubMed]
- Jeppesen SS, Schytte T, Brink C, et al. A Comparison of Stereotactic Body Radiation Therapy (SBRT) Versus No Treatment in Medically Inoperable Patients With Early-Stage Non-Small Cell Lung Cancer (NSCLC). Int J Radiat Oncol Biol Phys 2014;90:S642.
- White DC, D’Amico TA. Radiofrequency ablation for primary lung cancer and pulmonary metastases. Clin Lung Cancer 2008;9:16-23. [PubMed]
- Lencioni R, Crocetti L, Cioni R, et al. Radiofrequency ablation of lung malignancies: where do we stand? Cardiovasc Intervent Radiol 2004;27:581-90. [PubMed]
- Sharma A, Abtin F, Shepard JA. Image-guided ablative therapies for lung cancer. Radiol Clin North Am 2012;50:975-99. [PubMed]
- Beland MD, Wasser EJ, Mayo-Smith WW, et al. Primary non-small cell lung cancer: review of frequency, location, and time of recurrence after radiofrequency ablation. Radiology 2010;254:301-7. [PubMed]
- Lanuti M, Sharma A, Digumarthy SR, et al. Radiofrequency ablation for treatment of medically inoperable stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:160-6. [PubMed]
- Bi N, Shedden K, Zheng X, et al. Comparison of the Effectiveness of Radiofrequency Ablation With Stereotactic Body Radiation Therapy in Inoperable Stage I Non-Small Cell Lung Cancer: A Systemic Review and Meta-analysis. Pract Radiat Oncol 2013;3:S19. [PubMed]
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics 2005;25 Suppl 1:S69-83. [PubMed]
- Wolf FJ, Grand DJ, Machan JT, et al. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology 2008;247:871-9. [PubMed]
- Niu L, Xu K, Mu F. Cryosurgery for lung cancer. J Thorac Dis 2012;4:408-19. [PubMed]
- Vergnon JM, Huber RM, Moghissi K. Place of cryotherapy, brachytherapy and photodynamic therapy in therapeutic bronchoscopy of lung cancers. Eur Respir J 2006;28:200-18. [PubMed]
- Yamauchi Y, Izumi Y, Hashimoto K, et al. Percutaneous cryoablation for the treatment of medically inoperable stage I non-small cell lung cancer. PLoS One 2012;7:e33223. [PubMed]
- Simone CB 2nd, Friedberg JS, Glatstein E, et al. Photodynamic therapy for the treatment of non-small cell lung cancer. J Thorac Dis 2012;4:63-75. [PubMed]
- Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin 2011;61:250-81. [PubMed]
- Dougherty TJ, Gomer CJ, Henderson BW, et al. Photodynamic therapy. J Natl Cancer Inst 1998;90:889-905. [PubMed]
- Vrouenraets MB, Visser GW, Snow GB, et al. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer Res 2003;23:505-22. [PubMed]
- Furuse K, Fukuoka M, Kato H, et al. A prospective phase II study on photodynamic therapy with photofrin II for centrally located early-stage lung cancer. The Japan Lung Cancer Photodynamic Therapy Study Group. The Japan Lung Cancer Photodynamic Therapy Study Group. J Clin Oncol 1993;11:1852-7. [PubMed]
- Kato H, Usuda J, Okunaka T, et al. Basic and clinical research on photodynamic therapy at Tokyo Medical University Hospital. Lasers Surg Med 2006;38:371-5. [PubMed]
- Jeremic B, Classen J, Bamberg M. Radiotherapy alone in technically operable, medically inoperable, early-stage (I/II) non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2002;54:119-30. [PubMed]
- Sibley GS. Radiotherapy for patients with medically inoperable Stage I nonsmall cell lung carcinoma: smaller volumes and higher doses--a review. Cancer 1998;82:433-8. [PubMed]
- Qiao X, Tullgren O, Lax I, et al. The role of radiotherapy in treatment of stage I non-small cell lung cancer. Lung Cancer 2003;41:1-11. [PubMed]
- Mehta M, Scrimger R, Mackie R, et al. A new approach to dose escalation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2001;49:23-33. [PubMed]
- Fowler JF, Chappell R. Non-small cell lung tumors repopulate rapidly during radiation therapy. Int J Radiat Oncol Biol Phys 2000;46:516-7. [PubMed]
- Martel MK, Ten Haken RK, Hazuka MB, et al. Estimation of tumor control probability model parameters from 3-D dose distributions of non-small cell lung cancer patients. Lung Cancer 1999;24:31-7. [PubMed]
- Kavanagh BD, McGarry RC, Timmerman RD. Extracranial radiosurgery (stereotactic body radiation therapy) for oligometastases. Semin Radiat Oncol 2006;16:77-84. [PubMed]
- Videtic G, Hu C, Singh A, et al. Radiation Therapy Oncology Group (RTOG) Protocol 0915: A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy (SBRT) Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2013;87:S3.
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [PubMed]
- Timmerman RD, Hu C, Michalski J, et al. Long-term Results of RTOG 0236: A Phase II Trial of Stereotactic Body Radiation Therapy (SBRT) in the Treatment of Patients with Medically Inoperable Stage I Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2014;90:S30.
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [PubMed]
- Haasbeek CJ, Lagerwaard FJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol 2011;6:2036-43. [PubMed]
- Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 2010;37:4078-101. [PubMed]
- Chang JY, Li QQ, Xu QY, et al. Stereotactic ablative radiation therapy for centrally located early stage or isolated parenchymal recurrences of non-small cell lung cancer: how to fly in a “no fly zone”. Int J Radiat Oncol Biol Phys 2014;88:1120-8. [PubMed]
- Nyman J, Hallqvist A, Lung JA, et al. SPACE - A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. ESTRO 2014. Vienna, Austrian, 2014;abstr: OC-0565.
- Bogart JA, Hodgson L, Seagren SL, et al. Phase I study of accelerated conformal radiotherapy for stage I non-small-cell lung cancer in patients with pulmonary dysfunction: CALGB 39904. J Clin Oncol 2010;28:202-6. [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [PubMed]
- The ASCO Post. Study Projects Nationwide Low-Dose CT Screening Will Identify More—and Earlier-Stage—Lung Cancers, but Comes With Substantial Medicare Costs. Available online: http://www.ascopost.com/ViewNews.aspx?nid=16223, accessed on 22 June 2015.
- Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg 2014;97:1901-6; discussion 1906-7.
- Shirvani SM, Jiang J, Chang JY, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys 2012;84:1060-70. [PubMed]
- Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol 2010;28:928-35. [PubMed]
- Palma D, Lagerwaard F, Rodrigues G, et al. Curative treatment of Stage I non-small-cell lung cancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review. Int J Radiat Oncol Biol Phys 2012;82:1149-56. [PubMed]
- Verstegen NE, Oosterhuis JW, Palma DA, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol 2013;24:1543-8. [PubMed]
- Zheng X, Schipper M, Kidwell K, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys 2014;90:603-11. [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [PubMed]
- Zemlyak A, Moore WH, Bilfinger TV. Comparison of survival after sublobar resections and ablative therapies for stage I non-small cell lung cancer. J Am Coll Surg 2010;211:68-72. [PubMed]
- Kwan SW, Mortell KE, Talenfeld AD, et al. Thermal ablation matches sublobar resection outcomes in older patients with early-stage non-small cell lung cancer. J Vasc Interv Radiol 2014;25:1-9.e1.
- Bilal H, Mahmood S, Rajashanker B, et al. Is radiofrequency ablation more effective than stereotactic ablative radiotherapy in patients with early stage medically inoperable non-small cell lung cancer? Interact Cardiovasc Thorac Surg 2012;15:258-65. [PubMed]
- Kodama H, Yamakado K, Takaki H, et al. Lung radiofrequency ablation for the treatment of unresectable recurrent non-small-cell lung cancer after surgical intervention. Cardiovasc Intervent Radiol 2012;35:563-9. [PubMed]
- Sher DJ, Wee JO, Punglia RS. Cost-effectiveness analysis of stereotactic body radiotherapy and radiofrequency ablation for medically inoperable, early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2011;81:e767-74. [PubMed]


