Editor’s note:
In the era of personalized medicine, a critical appraisal new developments and controversies are essential in order to derived tailored approaches. In addition to its educative aspect, we expect these discussions to help younger researchers to refine their own research strategies.
Cons: long-term CT-scan follow-up is not the standard of care in patients curatively treated for an early stage non-small cell lung cancer
Introduction
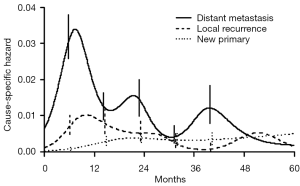
About 25% of patients with early stage (I, II, IIIA non-N2) non-small cell lung cancer (NSCLC) qualify for a treatment with curative intent, consisting of either radical surgical resection or radical radiotherapy. The former consists of at least an anatomical lobectomy, the latter is nowadays mainly given at ablative doses with stereotactic techniques (SABR). Radically treated patients may develop either locally or distantly relapsing lung cancer, or a second primary (lung) cancer. Besides, they retain a significant excess conditional mortality with an increasing relative contribution of cardiovascular and respiratory co-morbidity (1). Recurrence dynamics of resected early-stage NSCLC displays a multi-peak pattern, which supports the hypothesis of a metastasis growth model previously described for early-stage breast cancer (2). An initial surge in the hazard rate 9 months after surgery, is followed by two smaller peaks at the end of the second and fourth years, respectively (Figure 1). This pattern is dominated by distant metastatic events which decrease over time and are virtually absent after 5 years. Two distinguishable peaks are noted for local recurrence in the first and second years, but this is rare thereafter. The risk of local or distant recurrence is 10-38%, mainly dependent of stage and highest in pII-III NSCLC. This risk can be moderately reduced by the administration of postoperative platinum-based chemotherapy, with an average increase in 5-year survival of 5% (3). In contrast, the hazard rate for second primary lung cancer exhibits a more uniform pattern over time, is 1% to 4% per patient per year in most series (4) and increases even after 5 years. The median time interval between the two tumours is 14.5 months (5,6). Lastly, these patients are at risk of developing a second primary non-respiratory cancer: the most frequently diagnosed tumours are located in the head and neck and the urinary tract.
The outcome of recurrent lung cancer depends on the type of recurrence, its stage at diagnosis and residual functional treatment capacity. The stage of a second primary lung cancer is the strongest predictor of survival (7). Whereas the treatment of distant metastatic disease is palliative, some patients with loco-regional recurrence or second primary cancer benefit from a second curative treatment, either by resection or by SABR. This is highly dependent of the residual pulmonary function after the first treatment and cardiac co-morbidity. Only a fraction of patients with early stage recurrence are hence benefiting from a surveillance strategy.
Using a systematic postoperative surveillance protocol using CT and chest X-ray (CXR) over a 5-year period, 19 second primary lung cancers were diagnosed among 124 patients who had undergone previous resection, of whom 74% underwent a curative second resection (8,9). The 5-year survival of patients undergoing a reoperation for a second primary was between 25-60% (10-12) (Table 1). Only 1 of 9 isolated local recurrences was resectable, even though 8 of 9 recurrences were asymptomatic at the time of detection. Voltolini et al. reported that 5-year survival after reoperation for locally recurrent bronchogenic carcinoma was 15.5% (10). The 5-year post-recurrence survival in another series of patients undergoing reoperation after local recurrence was also 15% (13). In resected stage I NSCLC with local recurrence, a second surgical resection had a more favourable survival [hazard ratio (HR) 0.089)] than with chemotherapy and/or radiotherapy (HR 0.326) and without treatment (HR 1.0, reference; P = 0.001) (6,14). In early stages of recurrent or secondary lung carcinoma, even higher local control and overall survival rates can be achieved by complete pneumonectomy, with 5-year survival of about 50% in stage I and 40% in stage II carcinoma (15).
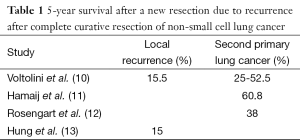
Full table
Short term surveillance after radical lung cancer treatment
Opinions differ and evidence is only moderately strong regarding the intensity and duration of surveillance strategy in the first years after a radical treatment. Resection rates for local-only initial recurrence of 33% and 70% are reported using CT for surveillance compared with 37.5% using CXR. Other series report resection rates for metachronous tumors of 63% and 75% using surveillance by CXR. Table 2 lists the available guidelines and recommendations with their grade of evidence. Some recommendations even change grade without proper new evidence, reflecting their expert’s rather than evidence-based decision process. Whereas most guidelines agree on periodical history, physical exam and CXR, variation is present on the frequency of chest CT-scan, varying from none over 4 monthly to yearly for life. We know that CT-scan is superior to CXR in the follow-up of patients after curative resection of lung cancer (22). Recurrences at the post-resection site were detected by CT-scan with a 94% sensitivity and 87% specificity, and a negative predictive value of 99%. Positive predictive value was only 53%. The abovementioned variation in surveillance intensity can be explained by differences in the reported outcomes, varying from detection of early recurrence over resectability, outcome and toxicity or complications of treatment. For second primary lung cancer, a better 5-year survival rate was reported in patients in whom a CT-scan surveillance was installed (18). In a retrospective cohort study using Surveillance, Epidemiology and End Results (SEER)-Medicare data to determine the imaging study used between 90-365 days following surgical resection in stage I-IIIA NSCLC between 1998 and 2009, the comparative effectiveness of CT-scan vs. CXR surveillance was explored in terms of overall survival (OS), using a stratified Cox model based on stage and adjusted for age, gender, race, census median income, Charlson comorbidity index, and adjuvant chemotherapy (23): 5,968 (54%) patients were followed by CT, and 5,083 (46%) by CXR. Patients with earlier stage, older age, and lower census median income were less likely to undergo CT surveillance. CT surveillance increased over the study period from 23% in 1998 to 68% in 2009. In the analysis of surveillance modality and OS, a significant interaction was identified between imaging and diagnosis year (P<0.001). The effect of CT surveillance on OS steadily improved over time, and was significantly better than CXR in the most recent time periods of study.
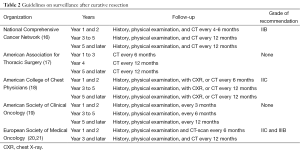
Full table
The schedule of follow up should be ideally modelled to cluster follow-up visits within recurrence peaks at 9 months, 2 and 4 years, to detect events at a time when they may be treated with curative intent (2). Most series do not report on quality of life or other patient-related outcomes. Walsh et al. illustrated that screening for asymptomatic recurrent lung cancer is unlikely to be cost effective (6). A French randomized study is currently addressing the issue of the intensity of surveillance during the first years post radical treatment (clintrials.gov NCT00198341). Pending these results, it is probably best to have at least one chest CT-scan performed within the first postoperative year in patients considered fit for further radical treatment.
Long term surveillance after radical lung cancer treatment
The issue whether surveillance should arbitrarily stop 5 years after treatment is increasingly challenged by recent data on lung cancer screening by low dose spiral CT-scan in a risk population of (ex-)smokers (24). Low-dose CT-scan seems to be comparable to standard-dose CT with regard to the identification of recurrent disease. The National Lung cancer Screening Trialists (NLST) found a 20 percent lower lung cancer mortality among trial participants screened with low-dose helical CT relative to CXR. In the randomized NELSON lung cancer screening trial using low dose spiral CT-scan, 5-year lung cancer survivors are eligible for enrolment in view of their increased risk of second primary lung cancer (25). Data on the prevalence of participants with a second primary cancer and their outcome are awaited.
Long term follow up of curatively treated early lung cancer patients is increasingly becoming an issue now that CT-scan screening will detect more patients in an early stage in whom survival is high and who are, independently of their smoking status, at risk of developing a second primary lung cancer 5 or more years after their first one. Although the data of the NLST are compelling and invite to implementation to the population of radically treated patients, several caveats argue against blind extrapolation:
- NLST and NELSON participants had to be eligible for radical resection. Increasing comorbidity and functional impairment by a previous resection will render patients less fit for surgery. Although SABR or sublobar resections could replace the standard anatomical lobectomy in lesser fit patients, their equivalence is still debated (26,27);
- Cost effectiveness (CE) of lung cancer screening in a risk population is estimated to be 81,000 $/QALY with the number of CT-scan being the main cost driver (28). As the incidence of non-calcified nodules in the population of radically treated NSCLC is likely higher than in the NLST, the number of confirmatory conventional dose CT-scan will be higher, negatively influencing the CE balance;
- Other drivers of CE of screening with low dose spiral CT are age, smoking status and gender. It is unclear whether the population of radically treated NSCLC will match with the NELSON and NLST population for these characteristics, making assumptions about the non-inferiority of low-dose CT-scan in survivors of lung cancer unlikely;
- How the CT-scan is interpreted also will have an effect. For instance, if radiologists use the new American College of Radiology Lung RADS reporting system, the false positivity rate will decrease by about 50% and could substantially decrease the number of follow-up CT-scan required, at the cost of sensitivity (29);
- We should be aware that not all screen detected lung cancers are in an early stage. A screening CT-scan looks for non-calcified pulmonary nodules in an asymptomatic at risk population while a diagnostic CT-scan is performed in a person who has a sign or symptom of disease. There is also the risk of unnecessary invasive studies and therapy for “overdiagnosed” lung cancer. Observational studies of screening for lung cancer with low-dose CT that preceded the NLST trial have estimated the extent of overdiagnosis to range between 13 and 27 percent (30,31).
Conclusions
Pending the answers to these questions, it is hap hazardous to embark on a routine follow-up with low dose CT-scan beyond 5 years in all radically treated lung cancer patients. We recommend an international effort to draft and accrue participants in a large scale randomized trial comparing long term surveillance with periodic low dose spiral CT-scan versus a to be agreed standard follow-up, which could consist in simple follow up with or without CXR.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Janssen-Heijnen ML, van Erning FN, De Ruysscher DK, et al. Variation in causes of death in patients with non-small cell lung cancer according to stage and time since diagnosis. Ann Oncol 2015;26:902-7. [PubMed]
- Demicheli R, Fornili M, Ambrogi F, et al. Recurrence dynamics for non-small-cell lung cancer: effect of surgery on the development of metastases. J Thorac Oncol 2012;7:723-30. [PubMed]
- Pisters KM, Le Chevalier T. Adjuvant chemotherapy in completely resected non-small-cell lung cancer. J Clin Oncol 2005;23:3270-8. [PubMed]
- Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst 1998;90:1335-45. [PubMed]
- Duchateau CS, Stokkel MP. Second primary tumors involving non-small cell lung cancer: prevalence and its influence on survival. Chest 2005;127:1152-8. [PubMed]
- Walsh GL, O'Connor M, Willis KM, et al. Is follow-up of lung cancer patients after resection medically indicated and cost-effective? Ann Thorac Surg 1995;60:1563-70; discussion 1570-2. [PubMed]
- Mollberg NM, Ferguson MK. Postoperative surveillance for non-small cell lung cancer resected with curative intent: developing a patient-centered approach. Ann Thorac Surg 2013;95:1112-21. [PubMed]
- Endo C, Sakurada A, Notsuda H, et al. Results of long-term follow-up of patients with completely resected non-small cell lung cancer. Ann Thorac Surg 2012;93:1061-8. [PubMed]
- Sugimura H, Nichols FC, Yang P, et al. Survival after recurrent nonsmall-cell lung cancer after complete pulmonary resection. Ann Thorac Surg 2007;83:409-17; discussioin 417-8.
- Voltolini L, Paladini P, Luzzi L, et al. Iterative surgical resections for local recurrent and second primary bronchogenic carcinoma. Eur J Cardiothorac Surg 2000;18:529-34. [PubMed]
- Hamaji M, Allen MS, Cassivi SD, et al. Surgical treatment of metachronous second primary lung cancer after complete resection of non-small cell lung cancer. J Thorac Cardiovasc Surg 2013;145:683-90; discussion 690-1. [PubMed]
- Rosengart TK, Martini N, Ghosn P, et al. Multiple primary lung carcinomas: prognosis and treatment. Ann Thorac Surg 1991;52:773-8; discussion 778-9. [PubMed]
- Hung JJ, Hsu WH, Hsieh CC, et al. Post-recurrence survival in completely resected stage I non-small cell lung cancer with local recurrence. Thorax 2009;64:192-6. [PubMed]
- Pairolero PC, Williams DE, Bergstralh EJ, et al. Postsurgical stage I bronchogenic carcinoma: morbid implications of recurrent disease. Ann Thorac Surg 1984;38:331-8. [PubMed]
- Regnard JF, Icard P, Magdeleinat P, et al. Completion pneumonectomy: experience in eighty patients. J Thorac Cardiovasc Surg 1999;117:1095-101. [PubMed]
- National Comprehensive Cancer Network (NCCN) Guidelines in Oncology: Non-Small Cell Carcinoma. Available online: http://www.nccn.org/professionals/physician_gls/PDF/nscl.pdf, accessed on March 4, 2015.
- Jacobson FL, Austin JH, Field JK, et al. Development of The American Association for Thoracic Surgery guidelines for low-dose computed tomography scans to screen for lung cancer in North America: recommendations of The American Association for Thoracic Surgery Task Force for Lung Cancer Screening and Surveillance. J Thorac Cardiovasc Surg 2012;144:25-32. [PubMed]
- Colt HG, Murgu SD, Korst RJ, et al. Follow-up and surveillance of the patient with lung cancer after curative-intent therapy: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e437S-54S.
- Pfister DG, Johnson DH, Azzoli CG, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol 2004;22:330-53. [PubMed]
- Crinò L, Weder W, van Meerbeeck J, et al. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v103-15. [PubMed]
- Vansteenkiste J, Crinò L, Dooms C, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol 2014;25:1462-74. [PubMed]
- Nakamura R, Kurishima K, Kobayashi N, et al. Postoperative follow-up for patients with non-small cell lung cancer. Onkologie 2010;33:14-8. [PubMed]
- Ciunci CA, Paulson EC, Mitra N, et al. Patterns and effectiveness of surveillance after curative intent surgery in stage I-IIIA non-small cell lung cancer. J Clin Oncol 2015;33:abstr 7546.
- National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [PubMed]
- Horeweg N, Scholten ET, de Jong PA, et al. Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol 2014;15:1342-50. [PubMed]
- Treasure T, Rintoul RC, Macbeth F. SABR in early operable lung cancer: time for evidence. Lancet Oncol 2015;16:597-8. [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [PubMed]
- Black WC, Gareen IF, Soneji SS, et al. Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med 2014;371:1793-802. [PubMed]
- American College of Radiology. Lung Imaging Reporting and Data System (Lung-RADS). April 29, 2014. Available online: http://www.acr.org/Quality-Safety/Resources/LungRADS
- Sone S, Nakayama T, Honda T, et al. Long-term follow-up study of a population-based 1996-1998 mass screening programme for lung cancer using mobile low-dose spiral computed tomography. Lung Cancer 2007;58:329-41. [PubMed]
- Lindell RM, Hartman TE, Swensen SJ, et al. Five-year lung cancer screening experience: CT appearance, growth rate, location, and histologic features of 61 lung cancers. Radiology 2007;242:555-62. [PubMed]


