Treatment of advanced squamous cell carcinoma of the lung: a review
Not only is lung cancer the most commonly diagnosed cancer internationally, representing approximately 17% of new cancer diagnoses worldwide, but it also bears the highest mortality rate among all cancers (24% of cancer-related mortality worldwide) (1). In the United States (US), lung cancer is the second most commonly diagnosed cancer with an estimated 224,000 new cases in 2014 and remains the leading cause of cancer death in the US (2,3). Of these lung cancer cases, over 85% of them are classified as non-small cell lung cancer (NSCLC), with squamous cell carcinoma (SCC) of the lung comprising approximately 30% (4).
Nearly 80% of all lung cancer cases in men and 90% of cases in women are associated with smoking (5,6). SCC is most strongly associated with smoking in a dose-dependent manner, with one study finding that 91% of SCC was attributed to cigarette smoking (7-9).
With the exception of the newly approved nivolumab, there have been no other US Food and Drug Administration (FDA) approvals specifically for SCC of the lung. Moreover, driver mutations/rearrangements connected with FDA-approved agents in the epidermal growth factor receptor (EGFR) and echinoderm microtubule associated protein like 4—anaplastic lymphoma kinase (EML4-ALK) are very rarely associated with squamous cell histology. Recently, however, molecular genotyping has led to the application of targeted agents for mutations prevalent in SCC. This overview of the treatment of squamous cell lung carcinoma highlights these recent molecular advances and discusses applications of newer cytotoxic and targeted agents evaluated for the treatment of advanced SCC (Figure 1).
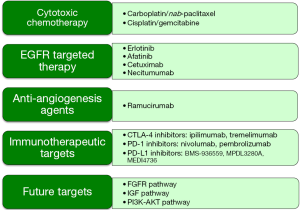
Cytotoxic chemotherapy
Cytotoxic chemotherapy for NSCLC has reached a therapeutic plateau as evidenced by the published data from Eastern Cooperative Oncology Group (EGOG) 1594 showing equivalent survivals among four different platinum doublet chemotherapies, with outcomes not analyzed by histology (10). Subsequent published data of a large phase III trial of cisplatin/pemetrexed versus cisplatin/gemcitabine, however, did indicate a difference in outcome based on histology (11). In this non-inferiority trial, patients with squamous cell histology received a relative benefit with the treatment of gemcitabine/cisplatin versus pemetrexed/cisplatin. Additional studies identified outcome discrepancies based on histology; a retrospective analysis of a phase III second-line trial revealed inferior survival in squamous cell cancer patients receiving pemetrexed compared with docetaxel and a phase III pemetrexed maintenance trial showed no benefit with pemetrexed maintenance in the squamous cell histologic subset (12,13). Based on the consistency of results across multiple trials indicating shorter survival in those with squamous histology, pemetrexed is not recommended for the treatment of patients with SCC (14).
Recently a large phase III trial comparing carboplatin/paclitaxel (solvent-based) to carboplatin/nab-paclitaxel (albumin bound) in stage IIIB and IV NSCLC also found a difference in efficacy based on histology. Though the two arms of the trial had similar survival outcomes, the nab-paclitaxel arm had an improved response (the primary endpoint of the trial) compared to the solvent-based paclitaxel arm; however, this benefit was limited to the SCC subset. The SCC subset exhibited a 41% radiologic response in the nab-paclitaxel arm compared to a 24% radiologic response in the solvent-based paclitaxel arm. Compared to the solvent-based paclitaxel group, the nab-paclitaxel group exhibited a numerically higher median overall survival in SCC (10.7 vs. 9.5 months) yet this was not statistically significant (HR 0.89, 95% CI, 0.719-1.101, P=0.284). In addition, the side effect profile in the nab-paclitaxel arm was more favorable, with less myalgias, neuropathy, and cytopenias (15). Ongoing studies should clarify the role of nab-paclitaxel in the treatment of squamous cell lung cancer patients (NCT identifier 02328105) (16). The lower toxicity profile has also bolstered its role as a potential agent in the maintenance setting (NCT identifier 02027428) (17).
EGFR targeted therapy
In patients with an EGFR activating gene mutation, there is ample evidence to offer first line EGFR tyrosine kinase inhibition (TKI) based on improved progression free survival and overall survival compared with cytotoxic chemotherapy (18-27). EGFR activating gene mutations are found in approximately 20% of adenocarcinomas but the prevalence in squamous cell cancers is considerably less (28). A study from Rekhtman et al. in 2012 illustrated that EGFR mutations do not occur in pure SCCs but appear only in mixed adeno-squamous carcinomas (29).
Though the response rate in patients without EGFR activating mutations is low, recent data may support the use of EGFR TKIs for later lines of therapy in wild type patients, including those with SCC (18). A retrospective study examining erlotinib in patients with advanced SCC found that of the 92 patients analyzed (74 of whom were current or former smokers), 16 achieved a partial response and 9 had stable disease. However, only 27 patients actually had molecular analysis performed on tumor specimens, and 2 were found to have EGFR complex mutations (30). The SATURN trial examining the efficacy of erlotinib as maintenance treatment in advanced NSCLC revealed that erlotinib prolonged progression free survival compared to placebo in both EGFR mutation-positive and EGFR mutation-negative tumors. The squamous cell subset analysis failed to reach statistical significance (31). The TAILOR trial comparing erlotinib to docetaxel as second-line treatment of patients with wild-type EGFR stage IV NSCLC showed that docetaxel was more effective than erlotinib (median overall survival was 8.2 months for docetaxel versus 5.4 months for erlotinib, and results trended in a similar direction for the SCC subset) (32).
It is possible that with a favorable proteomic signature, patients with wild-type EGFR tumors may have similar overall survival when treated with second-line chemotherapy or erlotinib as presented in the PROSE study using the VeriStrat test. Squamous cell patients were equally represented in both arms of the study (33). The ongoing LUX-Lung 8 trial is a prospective phase III trial comparing EGFR TKIs (afatinib vs. erlotinib) in patients with relapsed/refractory stage IIIB or IV SCC with ECOG performance status of 0-1 who had progressed after at least four cycles of platinum-based doublet chemotherapy and had not received prior EGFR TKI. Preliminary data suggest that the median progression free survival and disease control rate are higher for afatinib compared to erlotinib (2.7 vs. 1.9 months; 45.7% vs. 36.8%, respectively). This is tempered by higher incidences of diarrhea and stomatitis with afatinib (34).
Monoclonal antibodies against EGFR have shown moderate activity in NSCLC. Cetuximab, a recombinant human/mouse chimeric monoclonal antibody against EGFR, showed only minimal survival benefit when combined with cisplatin and vinorelbine (vs. chemotherapy alone) in a subset of patients with SCC (9 vs. 8.2 months), but this subgroup analysis did not reach statistical significance (35). Necitumumab, an IgG1 monoclonal antibody against EGFR, did not show any evidence that its addition to cisplatin/pemetrexed increased survival in first-line treatment of metastatic non-squamous NSCLC (36). However, outcomes were different when necitumumab was combined with different chemotherapy in a different histologic subset. The addition of necitumumab statistically improved overall survival, progression free survival, and disease control rate when added to cisplatin/gemcitabine in a trial conducted in SCC patients with a median overall survival improvement of 11.5 vs. 9.9 months (HR =0.84, P=0.012) (37).
Anti-angiogenesis agents
Bevacizumab, a VEGF inhibitor, has shown efficacy in NSCLC but is not recommended for SCC as it has been associated with life-threatening hemoptysis when used in SCC (38,39). Ramucirumab, a VEGFR2 inhibitor, was recently approved for second-line therapy for stage IV NSCLC based on results from the REVEL trial. The study compared ramucirumab/docetaxel to placebo/docetaxel in patients who progressed on platinum-based chemotherapy. Median overall survival was better (HR 0.86, 95% CI, 0.75-0.98, P=0.023) in the ramucirumab arm (10.5 months) compared to the placebo arm (9.1 months). Median progression free survival was also superior in the ramucirumab arm (4.5 vs. 3 months, P<0.0001). The study was not powered for subgroup analysis, though SCC patients made up approximately 25% of the trial and experienced a numeric improvement in median overall survival in the ramucirumab arm (9.5 vs. 8.2 months in placebo arm, HR 0.88, 95% CI, 0.69-1.13) (40). Phase II data investigating ramucirumab with paclitaxel/carboplatin as first-line therapy for stage IIIB/IV NSCLC revealed 6-month progression free survival rate of 59%, though 85% of patients had adenocarcinoma and phase II randomized data in the front-line squamous cell population has not been presented (41).
Immunotherapeutic targets
Another potential avenue within the field of targeted therapy for SCC involves immune-checkpoint inhibition. Aberrancies in the HLA-A gene were frequently noted in SCC from the Cancer Genome Atlas Project, suggesting a prominent role for immune evasion for these cancers (34). Pathways further along in study include the programmed cell death ligand 1 (PD-L1) and programmed cell death-1 (PD-1) and the CTLA-4 pathway. Tumors attempt to escape surveillance and detection by expressing PD-L1, which in turn interacts with the PD-1 on T-cells. This interaction leads to suppression of the antitumor T-cell response. Novel therapies are being developed to disrupt this PD-1/PD-L1 checkpoint (Figure 2). Two such therapies are nivolumab and pembrolizumab, which are monoclonal antibodies against the PD-1 receptor on T-cells so as to unmask the dormant T-cell antitumor response (42-44). PD-L1 inhibitors (BMS-936559, MPDL3280A, and MEDI4736) are also in development. While PD-1 inhibitors have been most extensively tested in patients with melanoma, new data suggest efficacy in NSCLC as well (45,46). As of October 2014, pembrolizumab has achieved breakthrough therapy designation for EGFR- and ALK- rearrangement-negative NSCLC following platinum-based chemotherapy, based on phase I results from the KEYNOTE-001 study. A total of 282 patients with treatment-naïve or previously treated advanced NSCLC were treated with pembrolizumab once every 3 weeks. The overall response rate (ORR) in the squamous histology group was 18-25% compared to 23% for the non-squamous histology group. At the time of publication of the data, only half of the patients had PD-L1 staining performed; of these, the ORR was 39-47% in patients with strong PD-L1 expression but only 9-16% in patients with weak/negative PD-L1 expression. The progression free survival and overall survival were also longer in patients with PD-L1 strong-positive patients. The median overall survival was found to be 8.2 months, while the median overall survival had not yet been reached in the treatment-naïve group (47).
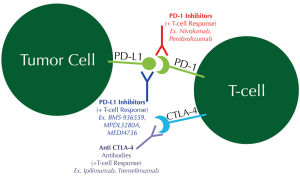
Nivolumab is still undergoing active trials. A prior phase II open-label, single-arm trial investigating the use of nivolumab in heavily pretreated patients with advanced squamous cell NSCLC (CheckMate-063) showed an 11-month ORR of 15% (95% CI, 9-22%), and all were partial responses. At the time of analysis, 10 of the 17 responding patients had response durations exceeding 6 months. This marks a key advancement over the previously demonstrated 1-year survival rates of 5.5-18% for third-line squamous cell NSCLC (48). A recent phase III trial of nivolumab compared to docetaxel as second-line therapy in patients with squamous cell NSCLC (CheckMate-017) was stopped early because of superior overall survival in the nivolumab arm (49). The 272 patients with advanced or metastatic SCC were randomized to either nivolumab or docetaxel after having progressed on prior platinum-based chemotherapy. The nivolumab arm experienced a 41% overall survival advantage over the docetaxel arm (9.2 vs. 6.0 months; HR 0.59, 95% CI, 0.44-0.79, P=0.00025). In contrast to the available pembrolizumab data, nivolumab exhibited improved overall survival compared with second line docetaxel regardless of PD-L1 immunohistochemistry expression (50). These milestone data were responsible for the recent expedited FDA approval of nivolumab specifically for the treatment of patients with advanced SCC who have progressed after platinum-based chemotherapy (51).
CTLA-4 inhibition has also been a topic of research in NSCLC. CTLA-4 is expressed by active cytotoxic T-cells, which acts as a negative regulatory molecule against T-cell response. These T-cells are silenced through interaction with ligands on antigen presenting cells. Anti-CTLA4 antibodies such as ipilimumab and tremelimumab bind to CTLA-4 thereby unleashing the antitumor effect of T-cells and increasing the ratio of effector T-cells to negative regulatory T-cells (52). In a phase II trial comparing the efficacy of paclitaxel/carboplatin alone (control arm) versus paclitaxel/carboplatin with ipilimumab (phased or concurrent) in stage IIIB and IV NSCLC, phased ipilimumab improved immune-related progression free survival (5.7 months for the phased ipilimumab arm vs. 4.6 months for the control arm). In comparison to non-squamous NSCLC, the SCC subgroup exhibited an even greater improvement in progression free survival with phased ipilimumab (53).
Future targets
Recent work by the Cancer Genomic Access Research Network has confirmed the complexity of SCC with a somatic mutation rate of 8.1 mutations per megabase, higher than other tumors studied including breast, glioblastoma, colorectal (54). There were only three cases of activating EGFR or KRAS mutations of 178 cases analyzed but the frequency of mutations predicted to have functional effect was over 50%. Targetable pathways such as PI3K/AKT, receptor tyrosine kinase and RAS had frequent alterations with at least one of those pathways altered in 69% of cases. The work also found previously identified targets such as fibroblast growth factor receptor (FGFR) 1 and PIK3CA (amplified in 20%), EPHA2 (mutated in 7%), MET (amplified in 6%), PDGFR (amplified in 8-10%), EGFR and AKT (mutated in 2-5%), some of which are highlighted below (42,55,56).
Fibroblast growth factor receptor (FGFR)
FGFR1 is a member of the FGFR tyrosine kinases, and activation is responsible for igniting the PI3K/AKT and RAS/MAPK pathways that stimulate growth and angiogenesis in several cancers (including SCC). FGFR1 is amplified in approximately 20% of SCC, and has shown to be associated with cigarette smoking in a dose-dependent fashion. There is some discordance as to whether FGFR1 amplification serves as a negative prognostic factor in surgically resected SCC with Kim et al. and a recent meta-analysis by Chang et al. supporting this assertion (55,57-60). Several FGFR inhibitors exist, including cediranib, nintedanib, pazopanib, and ponatinib (46). Cediranib is no longer under investigation given lack of efficacy in an early randomized trial (61). Nintedanib was studied with docetaxel (vs. docetaxel and placebo) in advanced NSCLC; overall survival in the nintedanib arm was only significantly improved in the adenocarcinoma patients but not in the total study population (62). Pazopanib (a dual FGFR and VFGFR inhibitor) was under investigation (NCT01208064, recently terminated early) but it has been limited by its heavy toxicity profile (63,64). Ponatinib is still undergoing trials (NCT01935336) but prior studies with head and neck cancer (NCT01761747) have been terminated due to toxicity (65). Novel non-ATP competitive FGFR1 inhibitors derived from nordihydroguaiaretic acid (NDGA) have shown promise in FGFR1 amplified SCC (66).
Insulin-like growth factor (IGF) pathway
The IGF pathway was recently a subject of interest, most notably with the IGF1R monoclonal antibody figitumumab. Initial phase II studies had suggested a benefit in SCC specifically, but two different phase III studies with figitumumab with either chemotherapy or erlotinib were prematurely ended due to excess toxicity and a lack of improvement in overall survival. Though this toxicity seemed to be correlated with low levels of circulating IGF, further progress in this pathway has been slow (55,67,68).
PI3-AKT signaling pathway
The PI3K-AKT signaling pathway is another potential candidate for targeted therapy. PIK3CA copy-number gains occur in 20% of all lung cancers, and frequency is even higher in SCC. PIK3CA mutations occur in approximately 6.5% of SCC. There are several PI3K inhibitors that are being actively developed; these include dual PI3K/MTOR inhibitors, isoform-selective PI3K inhibitors, and pan-PI3K inhibitors (55,69,70).
Conclusions
Lung cancer remains the single deadliest cancer both in the US and worldwide. The great majority of SCC is attributed to cigarette smoking, which fortunately is declining alongside cancer incidence. While we have been at a therapeutic plateau for advanced squamous cell lung cancer patients for several decades, recent observations suggest that we are on the verge of seeing incremental survival improvements for this relatively large group of patients. Current studies have confirmed an expanding role for immunotherapy, a potential opportunity for VEGFR inhibition, and even future targets in FGFR and PI3K-AKT that collectively should improve survival as well as quality of life for those affected by squamous cell lung cancer over the next decade.
Acknowledgements
None.
Footnote
Conflicts of Interest: Benjamin A. Derman, Kathryn F. Mileham, and Marta Batus have nothing to disclose. Philip D. Bonomi discloses clinical trial support from Eli Lilly, Bristol Myers Squibb, and Merck, as well as honoraria for advisory boards from Eli Lilly and Merck. Mary J. Fidler discloses consulting fees from Celgene and Bristol Myers Squibb.
References
- World Health Organization, International Agency for Research on Cancer. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available online: http://globocan.iarc.fr/Default.aspx, accessed on Jan 27 2015.
- Henley SJ, Richards TB, Underwood JM, et al. Lung cancer incidence trends among men and women--United States, 2005-2009. MMWR Morb Mortal Wkly Rep 2014;63:1-5. [PubMed]
- American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society, 2014.
- Heist RS, Mino-Kenudson M, Sequist LV, et al. FGFR1 amplification in squamous cell carcinoma of the lung. J Thorac Oncol 2012;7:1775-80. [PubMed]
- Pass HI, Carbone DP, Johnson DH, et al. Principles and Practice of Lung Cancer: The Official Reference Text of the International Association for the Study of Lung Cancer (IASLC). Philadelphia: Lippincott Williams & Wilkins, 2012.
- Barbone F, Bovenzi M, Cavallieri F, et al. Cigarette smoking and histologic type of lung cancer in men. Chest 1997;112:1474-9. [PubMed]
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [PubMed]
- Alberg AJ, Brock MV, Samet JM. Epidemiology of lung cancer: looking to the future. J Clin Oncol 2005;23:3175-85. [PubMed]
- Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw 2013;11:645-53. [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [PubMed]
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [PubMed]
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97. [PubMed]
- Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009;374:1432-40. [PubMed]
- ALIMTA (pemetrexed for injection) [package insert]. Indianapolis: Eli Lilly and Company, 2013.
- Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 2012;30:2055-62. [PubMed]
- LCI-LUN-ABR-001: Carbo With Nab-Paclitaxel in Patients With Advanced NSCL Cancer. ClinicalTrials.gov Identifier: NCT02328105. Available online: https://clinicaltrials.gov/ct2/show/NCT02328105, accessed on Feb 18 2015.
- Safety and Efficacy Study of Abraxane as Maintenance Treatment After Abraxane Plus Carboplatin in 1st Line Stage IIIB / IV Squamous Cell Non-small Cell Lung Cancer (aboundsqm). ClinicalTrials.gov Identifier: NCT02027428. Available online: https://www.clinicaltrials.gov/ct2/show/NCT02027428, accessed on Feb 18 2015.
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [PubMed]
- Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122-8. [PubMed]
- Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54-9. [PubMed]
- Gao G, Ren S, Li A, et al. Epidermal growth factor receptor-tyrosine kinase inhibitor therapy is effective as first-line treatment of advanced non-small-cell lung cancer with mutated EGFR: A meta-analysis from six phase III randomized controlled trials. Int J Cancer 2012;131:E822-9. [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [PubMed]
- D'Angelo SP, Pietanza MC, Johnson ML, et al. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol 2011;29:2066-70. [PubMed]
- Rekhtman N, Paik PK, Arcila ME, et al. Clarifying the spectrum of driver oncogene mutations in biomarker-verified squamous carcinoma of lung: lack of EGFR/KRAS and presence of PIK3CA/AKT1 mutations. Clin Cancer Res 2012;18:1167-76. [PubMed]
- Tseng JS, Yang TY, Chen KC, et al. Retrospective study of erlotinib in patients with advanced squamous lung cancer. Lung Cancer 2012;77:128-33. [PubMed]
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521-9. [PubMed]
- Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol 2013;14:981-8. [PubMed]
- Gregorc V, Novello S, Lazzari C, et al. Predictive value of a proteomic signature in patients with non-small-cell lung cancer treated with second-line erlotinib or chemotherapy (PROSE): a biomarker-stratified, randomised phase 3 trial. Lancet Oncol 2014;15:713-21. [PubMed]
- Goss G, Lu S, Felip E, et al. LUX-Lung 8: a randomized, open-label, phase III trial of afatinib vs. erlotinib in patients with advanced squamous cell carcinoma of the lung as second-line therapy following first-line platinum-based chemotherapy. Ann Oncol 2012;23:abstract 1477. Available online: http://oncologypro.esmo.org/Meeting-Resources/ESMO-2012/LUX-Lung-8-a-randomized-open-label-phase-III-trial-of-afatinib-vs.-erlotinib-in-patients-with-advanced-squamous-cell-carcinoma-of-the-lung-as-second-line-therapy-following-first-line-platinum-based-chemotherapy
- Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009;373:1525-31. [PubMed]
- Paz-Ares L, Mezger J, Ciuleanu TE, et al. Necitumumab plus pemetrexed and cisplatin as first-line therapy in patients with stage IV non-squamous non-small-cell lung cancer (INSPIRE): an open-label, randomised, controlled phase 3 study. Lancet Oncol 2015;16:328-37. [PubMed]
- Thatcher N, Hirsch FR, Szczesna A, et al. A randomized, multicenter, open-label, phase III study of gemcitabine-cisplatin (GC) chemotherapy plus necitumumab (IMC-11F8/LY3012211) versus GC alone in the first-line treatment of patients (pts) with stage IV squamous non-small cell lung cancer (sq-NSCLC). J Clin Oncol 2014;32:abstr 8008^.
- Socinski MA, Evans T, Gettinger S, et al. Treatment of stage IV non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e341S-68S.
- Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004;22:2184-91. [PubMed]
- Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665-73. [PubMed]
- Camidge DR, Berge EM, Doebele RC, et al. A phase II, open-label study of ramucirumab in combination with paclitaxel and carboplatin as first-line therapy in patients with stage IIIB/IV non-small-cell lung cancer. J Thorac Oncol 2014;9:1532-9. [PubMed]
- Chen Z, Fillmore CM, Hammerman PS, et al. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 2014;14:535-46. [PubMed]
- Errico A. Immunotherapy: PD-1-PD-L1 axis: efficient checkpoint blockade against cancer. Nat Rev Clin Oncol 2015;12:63. [PubMed]
- Al-Farsi A, Ellis PM. Treatment paradigms for patients with metastatic non-small cell lung cancer, squamous lung cancer: first, second, and third-line. Front Oncol 2014;4:157. [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [PubMed]
- Vincent MD. Promising targets and current clinical trials in metastatic squamous cell lung cancer. Front Oncol 2014;4:320. [PubMed]
- Garon EB, Gandhi L, Rizvi N, et al. LBA43 - Antitumor activity of pembrolizumab (Pembro; MK-3475) and correlation with programmed death ligand 1 (PD-L1) expression in a pooled analysis of patients (pts) with advanced non–small cell lung carcinoma (NSCLC). Ann Oncol 2014;25:1-41.
- Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [PubMed]
- Bristol-Myers Squibb. CheckMate-017, A Phase 3 Study of Opdivo (Nivolumab) Compared to Docetaxel in Patients with Second-Line Squamous Cell Non-small Cell Lung Cancer, Stopped Early. Available online: http://news.bms.com/press-release/checkmate-017-phase-3-study-opdivo-nivolumab-compared-docetaxel-patients-second-line-s
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [PubMed]
- Bristol-Myers Squibb. FDA Approves Opdivo (nivolumab) for the Treatment of Patients with Previously Treated Metastatic Squamous Non-Small Cell Lung Cancer. Available online: http://news.bms.com/press-release/fda-approves-opdivo-nivolumab-treatment-patients-previously-treated-metastatic-squamou?linkId=12694989, accessed on Mar 4 2015.
- Tomasini P, Khobta N, Greillier L, et al. Ipilimumab: its potential in non-small cell lung cancer. Ther Adv Med Oncol 2012;4:43-50. [PubMed]
- Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012;30:2046-54. [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519-25. [PubMed]
- Heist RS, Sequist LV, Engelman JA. Genetic changes in squamous cell lung cancer: a review. J Thorac Oncol 2012;7:924-33. [PubMed]
- Kenmotsu H, Serizawa M, Koh Y, et al. Prospective genetic profiling of squamous cell lung cancer and adenosquamous carcinoma in Japanese patients by multitarget assays. BMC Cancer 2014;14:786. [PubMed]
- Kim HR, Kim DJ, Kang DR, et al. Fibroblast growth factor receptor 1 gene amplification is associated with poor survival and cigarette smoking dosage in patients with resected squamous cell lung cancer. J Clin Oncol 2013;31:731-7. [PubMed]
- Chang J, Liu X, Wang S, et al. Prognostic value of FGFR gene amplification in patients with different types of cancer: a systematic review and meta-analysis. PLoS One 2014;9:e105524. [PubMed]
- Craddock KJ, Ludkovski O, Sykes J, et al. Prognostic value of fibroblast growth factor receptor 1 gene locus amplification in resected lung squamous cell carcinoma. J Thorac Oncol 2013;8:1371-7. [PubMed]
- Cihoric N, Savic S, Schneider S, et al. Prognostic role of FGFR1 amplification in early-stage non-small cell lung cancer. Br J Cancer 2014;110:2914-22. [PubMed]
- Laurie SA, Solomon BJ, Seymour L, et al. Randomised, double-blind trial of carboplatin and paclitaxel with daily oral cediranib or placebo in patients with advanced non-small cell lung cancer: NCIC Clinical Trials Group study BR29. Eur J Cancer 2014;50:706-12. [PubMed]
- Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014;15:143-55. [PubMed]
- Scagliotti GV, Felip E, Besse B, et al. An open-label, multicenter, randomized, phase II study of pazopanib in combination with pemetrexed in first-line treatment of patients with advanced-stage non-small-cell lung cancer. J Thorac Oncol 2013;8:1529-37. [PubMed]
- Pazopanib Hydrochloride or a Placebo in Treating Patients With Non-Small Cell Lung Cancer Who Have Received First-Line Chemotherapy. ClinicalTrials.gov Identifier: NCT01208064. Available online: https://clinicaltrials.gov/ct2/show/record/NCT01208064, accessed on Feb 18 2015.
- Study of Ponatinib in Patients With Lung Cancer Preselected Using Different Candidate Predictive Biomarkers. ClinicalTrials.gov Identifier: NCT01935336. Available online: https://clinicaltrials.gov/ct2/show/study/NCT01935336, accessed on Feb 18 2015.
- Wu J, Ji J, Weng B, et al. Discovery of novel non-ATP competitive FGFR1 inhibitors and evaluation of their anti-tumor activity in non-small cell lung cancer in vitro and in vivo. Oncotarget 2014;5:4543-53. [PubMed]
- Langer CJ, Novello S, Park K, et al. Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer. J Clin Oncol 2014;32:2059-66. [PubMed]
- Scagliotti GV, Bondarenko I, Blackhall F, et al. Randomized, phase III trial of figitumumab in combination with erlotinib versus erlotinib alone in patients with nonadenocarcinoma nonsmall-cell lung cancer. Ann Oncol 2015;26:497-504. [PubMed]
- Lee H, Kim SJ, Jung KH, et al. A novel imidazopyridine PI3K inhibitor with anticancer activity in non-small cell lung cancer cells. Oncol Rep 2013;30:863-9. [PubMed]
- Papadimitrakopoulou V. Development of PI3K/AKT/mTOR pathway inhibitors and their application in personalized therapy for non-small-cell lung cancer. J Thorac Oncol 2012;7:1315-26. [PubMed]


