Whacking a mole-cule: clinical activity and mechanisms of resistance to third generation EGFR inhibitors in EGFR mutated lung cancers with EGFR-T790M
The mutational landscape of lung adenocarcinomas is complex and defined by heterogeneous subpopulations of tumors that can be addicted to oncogene-driven proliferative and anti-apoptotic signaling (1). Epidermal growth factor receptor (EGFR) mutations which were identified in 2004 (2-4)—are the poster children for the concept, as lung adenocarcinomas that harbor activating kinase domain EGFR mutations become addicted to deranged EGFR signaling and are susceptible to small-molecule compounds that disrupt EGFR activity (5). The clinically-relevant and most frequent EGFR mutations are inframe deletions/insertions (around amino-acid residues 747 to 752) of exon 19 (these account for up to 40-50% of all EGFR mutations) and the L858R mutation (this accounts for up to 30-40% of all EGFR mutations) of exon 21 (5-7). The transcribed EGFR mutant proteins favor the active kinase state, induce sustained mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinases (PI3K) cascades, resulting in hyperproliferative and anti-apoptotic cell phenotypes (5). Acute inhibition of EGFR through tyrosine kinase inhibitors (TKIs) in these oncogene addicted lung adenocarcinomas disrupts the intracellular signaling balance, leading to cell cycle arrest and apoptosis (8-10). The concept of “oncogene-addiction” (11) may be the shared basis of pathogenesis for all oncogenic kinase-driven tumors (12-14). These insights into the biology of EGFR mutations translated into the clinical real with the development of the first generation EGFR TKIs gefitinib and erlotinib, both of which are reversible ATP mimetic quinazoline derivatives (5,15,16); and also with the development of the second generation EGFR TKI afatinib, an irreversible inhibitor that binds to the C797 amino-acid residue of EGFR (17). First and second generation EGFR TKIs were originally developed to target the wild-type (WT) EGFR but are significantly more potent against common EGFR mutations and have a favorable therapeutic window (Figure 1) in tumors driven by EGFR-exon 19 deletions or EGFR-L858R (5). Over the last several years, a multitude of randomized clinical trials have compared an EGFR TKI (gefitinib, erlotinib or afatinib) against systemic platinum-based chemotherapies in advanced lung adenocarcinomas. In all of these trials, the response rates (RRs) with the EGFR TKIs exceeded 70% being >2 times higher than platinum-doublets, the median progression-free survival (PFS) times were significantly longer (with a median of approximately 10-12 months) than that with chemotherapy and the median overall survival (OS) times augmented to over 24 months, especially in tumors with EGFR-exon 19 deletions (18)—despite a high rate of cross-over from chemotherapy to EGFR TKI (1,15-17). The combined data from these studies now define the clinical management of EGFR mutated lung cancers. Erlotinib, gefitinib and afatinib are approved worldwide for the first line treatment of lung adenocarcinomas with EGFR-exon 19 deletions or EGFR-L858R mutations (5,18).
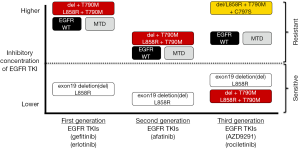
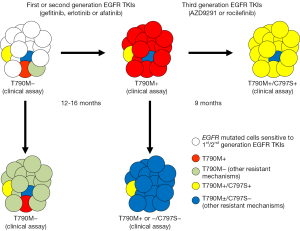
The advances brought forth by first and second generation EGFR TKIs not only validated EGFR as an important target for lung cancer but also highlighted some of the limitations of these EGFR TKIs. Acquired resistance to erlotinib/gefitinib and afatinib therapy can come about through multiple biological mechanisms that highlight tumor heterogeneity and adaptability [i.e., a game of whack-a-mole analogy (Figure 2)]: (I) the gatekeeper kinase EGFR-T790M mutation that modifies ATP affinity, drug binding properties and shifts inhibitory curves (5); (II) activation of bypass signaling cascades that reactivate the MAPK and PI3K downstream pathways (5); and (III) phenotypic and genomic neuroendocrine transformation that silences the expression of or dependence on EGFR protein (19-21). By far, the selection of tumors harboring the original activating EGFR mutation with concurrent EGFR-T790M is the most common (>50-60%) mechanism of acquired resistance to first/second generation of EGFR TKIs (5,22-26). We and others first identified EGFR-T790M in 2005 (22,27), which leads to a threonine (T) to a methionine (M) amino-acid change at the 790 “gatekeeper” regulatory position of the EGFR kinase (22,27). In addition to its effect on ATP affinity and drug binding (5), EGFR-T790M can stimulate other oncogenic signals—such as the β-catenin pathway (23). Originally, EGFR-T790M was reported as an acquired mutation after exposure to first generation EGFR TKIs; however, recent progress with sensitive sequencing technologies has revealed that pre-existing EGFR-T790M clones can be detected in patients with TKI-naïve tumors (28); as indicated in Figure 2. In this context, emergence of EGFR-T790M may be due to selection of “EGFR-T790M-positive” clones under pressure from a first/second generation EGFR TKI (Figure 2).
Since we first reported that an irreversible EGFR TKI can inhibit EGFR-T790M in vitro (29), efforts has been made to identify potent irreversible (i.e., C797-binding) EGFR TKIs to overcome resistance caused by EGFR-T790M. However, the initial selected clinical compounds (i.e., second generation EGFR TKIs such as afatinib and dacomitinib) failed to induce responses in the clinical acquired resistance to gefitinib/erlotinib setting (5). These disappointing results can be explained by lack of EGFR mutant selectivity of second generation EGFR TKIs and their inexistent therapeutic window towards EGFR-T790M when compared to WT EGFR (Figure 1). Afatinib and others in the same class are extremely potent WT EGFR inhibitors and achievable serum/plasma levels (limiting toxicities include skin and gastrointestinal adverse events) in patients are unable to inhibit EGFR-T790M bearing lung adenocarcinomas (5,6,30).
A major breakthrough in targeting EGFR-T790M occurred in 2009 with the identification of a novel class of covalent EGFR pyrimidine TKIs that are more selective for EGFR-T790M and EGFR TKI-sensitizing mutations than to WT EGFR (31). This class of TKIs against EGFR-T790M heralded the clinical development of third generation EGFR TKIs (Figure 1). The two compounds that have advanced the furthest are AZD9291 (AstraZeneca, with a proposed name of mereletinib) and rociletinib (Clovis Oncology, formerly named CO-1686). The impressive results from the expanded phase I first-in-human studies for both drugs were published in April 2015 (32,33). The phase I trial of AZD9291 (AURA) evaluated escalating doses of the drug in patients with advanced EGFR mutated lung cancer with resistance to treatment with the first generation EGFR TKIs (erlotinib/gefitinib) (32). A total of 253 patients were included in doses of AZD9291 of 20 mg up to 240 mg daily but a dose of 80 mg daily was considered as optimal to maximize efficacy and minimize skin/gastrointestinal adverse events observed at the higher doses (32). A total of 138 patients had tumors that were confirmed to harbor EGFR-T790M and 127 were evaluated for responses; with a RR of 61% (95% CI, 52-70%), disease control rate (DCR) of 95% (95% CI, 90-98%) and a median PFS of 9.6 months (95% CI, 8.3-not reached). As expected, tumors not harboring EGFR-T790M (61 evaluable patients) fared worse with a RR of 21% (95% CI, 12-34%), DCR of 61% (95% CI, 47-73%) and a dismal median PFS of 2.8 months (95% CI, 2.1-4.3 months). AZD9291 has been granted breakthrough therapy designation, orphan drug and fast track status by the United States Food and Drug Administration (FDA); and its approval with a companion diagnostic for EGFR-T790M is imminent based on results of the aforementioned AURA study and an ongoing phase II trial of AZD9291 80 mg daily for EGFR-T790M mutated lung adenocarcinomas (AURA-2 study). This third generation EGFR TKI is also being investigated in randomized trials after progression on gefitinib, erlotinib or afatinib against evidenced-based chemotherapies (AURA-3 study), as a first line therapy for EGFR mutated lung adenocarcinoma against gefitinib or erlotinib (FL-AURA study), and in combination with anti-PDL1 immunotherapies (MEDI4736), MEK inhibitors (selumetinib) or MET inhibitors (AZD6094) as part of the TATTON study. The phase I-II trial of rociletinib (TIGER-X) evaluated escalating doses of the drug in patients with advanced EGFR mutated lung cancer with acquired resistance to first or second generation EGFR TKIs (33). A total of 130 patients were enrolled and received escalating doses of free-base and subsequently hydrogen bromide salt (HBr) drug formulations, with therapeutic doses considered to encompass 900 mg twice daily of free-base and 625,750 or 1,000 mg twice daily of HBr rociletinib (33). A total of 46 patients had tumors that were confirmed to harbor EGFR-T790M and were evaluated for responses; with a RR of 59% (95% CI, 45-73%), DCR of 93% and a median PFS of 13.1 months (95% CI, 5.4-13.1 months). Tumors not harboring EGFR-T790M (17 evaluable patients) fared worse with a RR of 29% (95% CI, 8-51%), DCR of 59% and a median PFS of 5.6 months (95% CI, 1.3-not reached). Interestingly, the predominant grade 3 adverse event was hyperglycemia thought to be secondary to a rociletinib metabolite that inhibits the type I insulin-like growth factor receptor (33); and the latter adverse event (often requiring anti-diabetic medications) in addition to concerns related to cardiac QT prolongation may hamper the rapid clinical development of this drug. Rociletinib has been granted breakthrough therapy designation by the FDA with data from the aforementioned TIGER-X and a global registration phase II trial in EGFR-T790M positive lung adenocarcinomas (TIGER-2 study) being evaluated for safety plus efficacy. This third generation EGFR TKI is also being investigated in randomized trials after progression on gefitinib, erlotinib or afatinib against evidenced-based chemotherapies (TIGER-3 study) and as a first line therapy for EGFR mutated lung adenocarcinoma against gefitinib or erlotinib (TIGER-1 study).
Despite the thrilling responses seen with AZD9291 and rociletinib in lung adenocarcinomas with acquired resistance to gefitinib, erlotinib or afatinib harboring the recalcitrant EGFR-T790M mutation (32,33), it is painfully evident that tumor plasticity and selection pressure continue to drive tumor adaptation and resistance to third generation EGFR TKIs (Figure 2). The clinical investigators of the AZD9291 clinical trials have convincingly shown that biological mechanisms of resistance to this drug can be readily identified in cell-free plasma DNA from patients (34). The most frequent (40% of 15 EGFR-T790M cases treated with AZD9291 in the AURA study) mechanism identified was the acquisition of the EGFR-C797S mutation in exon 20 of EGFR. These investigators and other show in preclinical models that EGFR-exon 19 deletion + T790M + C797S and EGFR − L858R + T790M + C797S generate proteins that are resistant to AZD9291, rociletinib and all irreversible EGFR TKIs (including quinazolone- and pyrimidine-based compounds) by impairing covalent binding of these drugs to the C797 amino-acid residue of EGFR (34-36). Plasma samples also showed that another 33% of cases with AZD9291 progression only had EGFR-T790M and the original sensitizing mutation detected (Figure 2), and in another 27% of cases the EGFR-T790M was no longer detected (34). Although the plasma DNA was unable to evaluate for non-EGFR mutational mechanisms of acquired resistance, plentiful preclinical reports using third generations EGFR TKIs (including AZD9291) have consistently demonstrated bypass activation of the MAPK-ERK-RAS pathway (through MAPK1 amplification, downregulation of negative regulators of ERK, NRAS mutation/amplification, KRAS amplification among others) as a major escape valve to EGFR inhibition (37,38). The clinical investigators of the rociletinib clinical trials have also demonstrated similarly that resistance to rociletinib in the TIGER-X study can be accompanied by putative bypass mechanisms in the presence or absence of EGFR-T790M or EGFR-T790M amplification (39). In addition, their group also reported neuroendocrine transformation of adenocarcinomas to small cell lung cancer with genotypic/phenotypic silencing of EGFR protein expression as a mechanism of resistance in 16% (2/12 cases) of rociletinib re-biopsies (39). Future reports of tumor and liquid biopsies of lung adenocarcinomas resistant to third generation EGFR TKIs will help define the true frequency of EGFR-C797S, MAPK pathway activation and small cell transformation as mechanisms of resistance to this new class of TKI (Figure 2).
EGFR mutated NSCLCs (those with exon 19 deletions or L858R) have come a long way in the last decade. Most patients with a new diagnosis of advanced EGFR mutated NSCLC in 2015 can expect to receive multiple lines of monotherapy with first, second and third generation EGFR TKIs and can anticipate a median OS that exceeds 2-3 years, which is undoubtedly a tremendous success given that a median OS in pre-EGFR TKI era was less than 1 year. However, the use of monotherapies with EGFR TKIs has also underscored the painful reality that a relentless game of whack-a-mole is constantly being played between TKIs and a highly heterogeneous/adaptable cancer; with the lung adenocarcinoma eventually winning out through mutations (EGFR-T790M and/or C797S), bypass mechanisms or histologic/genotypic transformation (Figure 2). The next decade of research milestones for EGFR mutated lung adenocarcinomas will need to address current unmet clinical needs; which include: the role of first, second and third generation EGFR TKIs in the management of earlier stages (I-III) of NSCLC, the need for improved management of difficult-to-treat sanctuary sites such as the central nervous system (40), and the requisite for treatment strategies (most likely combination therapies with PI3K/MAPK inhibitors, immunotherapies or cytotoxic agents) that can delay or overcome acquired resistance to first, second and third generation EGFR inhibitors. We hope that we will eventually “catch all moles” (Figure 2) and “win” the game between TKIs and oncogenic kinase-driven tumors.
Acknowledgements
Funding: This work was funded in part through a Lung Cancer Foundation of America-International Association for the Study of Lung Cancer grant (to Daniel B. Costa), an American Cancer Society grant RSG 11-186 (to Daniel B. Costa), and National Cancer Institute grants CA090578 (to Daniel B. Costa), CA169259 (to Susumu S. Kobayashi) and CA178301 (to Susumu S. Kobayashi).
Footnote
Provenance: This is a Guest Editorial commissioned by the Section Editor Hongbing Liu (Department of Respiratory Medicine, Jinling Hospital, Nanjing University School of Medicine, Nanjing, China).
Conflicts of Interest: Daniel B. Costa has received consulting fees and honoraria from Pfizer Inc and Boehringer Ingelheim, respectively. Daniel B. Costa also conducts unremunerated clinical trials using AZD9291 (AstraZeneca) and rociletinib (Clovis Oncology). Susumu S. Kobayashi has received honoraria from Bristol-Myers Squibb. No other conflict of interest is stated.
References
- Gerber DE, Gandhi L, Costa DB. Management and future directions in non-small cell lung cancer with known activating mutations. Am Soc Clin Oncol Educ Book 2014.e353-65. [PubMed]
- Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101:13306-11. [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [PubMed]
- Jorge SE, Kobayashi SS, Costa DB. Epidermal growth factor receptor (EGFR) mutations in lung cancer: preclinical and clinical data. Braz J Med Biol Res 2014;47:929-39. [PubMed]
- Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol 2012;13:e23-31. [PubMed]
- Yasuda H, Park E, Yun CH, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med 2013;5:216ra177.
- Costa DB, Halmos B, Kumar A, et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med 2007;4:1669-79; discussion 1680.
- Cragg MS, Kuroda J, Puthalakath H, et al. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med 2007;4:1681-89; discussion 1690.
- Gong Y, Somwar R, Politi K, et al. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med 2007;4:e294. [PubMed]
- Sharma SV, Settleman J. Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes Dev 2007;21:3214-31. [PubMed]
- Will B, Siddiqi T, Jordà MA, et al. Apoptosis induced by JAK2 inhibition is mediated by Bim and enhanced by the BH3 mimetic ABT-737 in JAK2 mutant human erythroid cells. Blood 2010;115:2901-9. [PubMed]
- Kuroda J, Puthalakath H, Cragg MS, et al. Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc Natl Acad Sci U S A 2006;103:14907-12. [PubMed]
- Sale MJ, Cook SJ. The BH3 mimetic ABT-263 synergizes with the MEK1/2 inhibitor selumetinib/AZD6244 to promote BIM-dependent tumour cell death and inhibit acquired resistance. Biochem J 2013;450:285-94. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [PubMed]
- Lee CK, Wu YL, Ding PN, et al. Impact of Specific Epidermal Growth Factor Receptor (EGFR) Mutations and Clinical Characteristics on Outcomes After Treatment With EGFR Tyrosine Kinase Inhibitors Versus Chemotherapy in EGFR-Mutant Lung Cancer: A Meta-Analysis. J Clin Oncol 2015;33:1958-65. [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [PubMed]
- Niederst MJ, Sequist LV, Poirier JT, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun 2015;6:6377. [PubMed]
- Le X, Desai NV, Majid A, et al. De novo pulmonary small cell carcinomas and large cell neuroendocrine carcinomas harboring EGFR mutations: Lack of response to EGFR inhibitors. Lung Cancer 2015;88:70-3. [PubMed]
- Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786-92. [PubMed]
- Nakayama S, Sng N, Carretero J, et al. β-catenin contributes to lung tumor development induced by EGFR mutations. Cancer Res 2014;74:5891-902. [PubMed]
- Chong CR, Jänne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med 2013;19:1389-400. [PubMed]
- Ohashi K, Maruvka YE, Michor F, et al. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. J Clin Oncol 2013;31:1070-80. [PubMed]
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240-7. [PubMed]
- Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73. [PubMed]
- Watanabe M, Kawaguchi T, Isa SI, et al. Ultra-sensitive detection of the pretreatment EGFR T790M mutation in non-small-cell lung cancer patients with an EGFR-activating mutation using droplet digital PCR. Clin Cancer Res 2015;21:3552-60. [PubMed]
- Kobayashi S, Ji H, Yuza Y, et al. An alternative inhibitor overcomes resistance caused by a mutation of the epidermal growth factor receptor. Cancer Res 2005;65:7096-101. [PubMed]
- Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer 2009;10:281-9. [PubMed]
- Zhou W, Ercan D, Chen L, et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature 2009;462:1070-4. [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [PubMed]
- Sequist LV, Soria JC, Goldman JW, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med 2015;372:1700-9. [PubMed]
- Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015;21:560-2. [PubMed]
- Ercan D, Choi HG, Yun CH, et al. EGFR mutations and resistance to Irreversible pyrimidine based EGFR inhibitors. Clin Cancer Res 2015;21:3913-23. [PubMed]
- Niederst MJ, Hu H, Mulvey HE, et al. The allelic context of the C797S mutation acquired upon treatment with third generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies. Clin Cancer Res 2015;21:3924-33. [PubMed]
- Ercan D, Xu C, Yanagita M, et al. Reactivation of ERK signaling causes resistance to EGFR kinase inhibitors. Cancer Discov 2012;2:934-47. [PubMed]
- Eberlein CA, Stetson D, Markovets AA, et al. Acquired resistance to mutant-selective EGFR inhibitor AZD9291 is associated with increased dependence on RAS signaling in preclinical models. Cancer Res 2015;75:2489-500. [PubMed]
- Piotrowska Z, Niederst MJ, Karlovich CA, et al. Heterogeneity Underlies the Emergence of EGFR T790 Wild-Type Clones Following Treatment of T790M-Positive Cancers with a Third Generation EGFR Inhibitor. Cancer Discov 2015;5:713-22. [PubMed]
- Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015;88:108-11. [PubMed]


