Prognostic value of Twist in lung cancer: systematic review and meta-analysis
Introduction
Lung cancer is the leading cause of cancer mortality world-wide (1). Approximately 85% of all lung cancer is non-small cell lung cancer (NSCLC). Although great progress has been made in treatment recently, the prognosis of lung cancer is still poor. Cancer biomarkers can facilitate the early diagnosis and monitoring of the disease by contributing to our understanding of tumor biology and allowing more efficient therapeutic regimes to be applied earlier in the disease course, thus further improving patient survival (2). Several molecular markers have been reported to predict prognosis and survival of patients with NSCLC (3,4). The development of metastasis is the major obstacle to long-term survival. To identify the molecular markers related to metastasis may better predict the prognosis in patients.
The epithelial-mesenchymal transition (EMT), is a cellular process that epithelial cells lose their adhesion properties and acquire mesenchymal properties of migration and invasion (5,6). It allows polarized, immotile epithelial cells to convert to motile mesenchymal cells, with the acquisition of fibroblastoid morphology, enhanced potential for metastasis, resistance to apoptosis, and stem cell properties (5,7-9). The EMT is activated in a wide variety of cancer cells and enhanced potential for metastasis (6). Therefore, EMT is considered to play crucial role in inducing tumor invasion and metastasis (10,11). Repression of E-cadherin is a hallmark of the EMT process. Suppressed expression of E-cadherin leads to the loss of cell-cell adhesion and cancer progression (12). Based on the connection between EMT and cancer metastasis, the EMT regulatory molecules might be prognostic markers for NSCLC.
Transcription factors such as Twist, Snail, Zeb, and FoxC families are expressed in different cancers, and their overexpression induces EMT in a variety of cancer types (6,13). Among these, Twist is a basic helix-loop-helix transcription factor, which can promote EMT by down-regulating E-cadherin directly or interacting with other transcription factors (10,14,15). Twist has been reported to correlate with poor survival in a variety of carcinomas, such as breast, prostate, and hepatocellular carcinoma. Overexpression of Twist was detected in these tumors, and inhibition of Twist expression could significantly improve the survival (14-16). However, the prognostic value of Twist in lung cancer remains controversial. Miura et al. (17) suggested that Twist expression was not associated with EMT and might not predict the survival of NSCLC. However, Hung et al. (18) suggested an inverse correlation between Twist expression and patient prognosis. We therefore carried out a meta-analysis of data from published studies to quantitatively analyze the prognostic value of Twist in patients with lung cancer.
Materials and methods
Data mining
The Oncomine cancer microarray database was used to analyze the mRNA expression profiles of NSCLC tissues relative to their normal controls. The data mining strategy is based on a published methodology established by Oncomine (19). First, significantly upregulated genes in cancer samples compared with the normal tissue counterpart (greater than twofold, P<0.05) were selected. Then, concept filters in the Oncomine database were used to identify the known tyrosine kinases which differentially expressed in NSCLC.
Study selection and data extraction
A literature search of EMBASE, Medline, ISI Web of knowledge, and PubMed up to 5 January, 2015 was conducted. Search terms included “Twist”, “lung cancer”, “lung carcinoma”, “prognosis”, “survival”, “prognostic”, “outcome” and “significance”. The references reported in the identified studies were also used to complete the search. To avoid replicated data in different publications from the same author or the same researching team, we further checked these remaining articles.
The eligibility criteria in this meta-analysis as following: (I) trials had to conducted on human lung cancer; (II) the correlations between the expression of Twist and prognosis was reported; (III) Twist expression level was measured by immunohistochemistry (IHC); (IV) the hazard ratios (HRs) could be extracted directly or calculated indirectly; (V) published in English or Chinese.
Two reviewers (Zeng and Zhan) independently collected data from each study. Any lack of clarity and disagreements were resolved by discussion. A data extraction sheet based on the Cochrane Consumers and Communication Review Group’s data extraction template was developed. The following details were extracted from: first author, publication year, country of patients, sample number, histology, stage, first antibody, detective method, location of protein expression, high Twist rate, follow-up time and survival data.
Statistical analysis
To quantify the prognostic value of high Twist level, the survival analysis was conducted by comparing the high value to low value. The HRs and 95% confidence intervals (CIs) were directly extracted from the original studies or calculated from available survival by the Tierney’s methods if the data were not reported (20). Heterogeneity test with I2 statistic and Q statistic was performed (21). If HRs were found to have fine homogeneity, a fixed effect model was used for secondary analysis; if not, a random-effect model was used. HR >1 implies poor prognosis for high Twist level, and P<0.05 was considered to be statistically significant. We conducted subgroup analysis by ethnicity and histology, respectively. Sensitivity analyses were conducted to evaluate the stability of our results. Begg’s test and Egger’s test, and a Begg’s funnel plot were used to assess the publication bias. A value of “Pr > |z|” less than 0.05 or a value of “P> |t|” less than 0.05 was considered to be potential publication bias.
Results
Study selection and characteristics
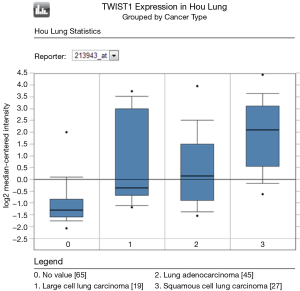
To determine whether Twist expression was associated with lung cancer in humans, we first examined Twist mRNA expression in the publicly available database Oncomine. SA; Oncomine was used for analysis and visualization). Compared with normal lung, Twist mRNA expression is significantly increased in lung cancer, especially in squamous cell cancer (Figure 1). We further explore the prognostic value of Twist by meta-analysis.
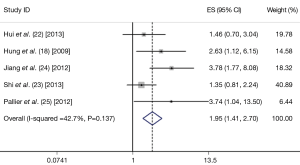
A total of five studies were included finally (18,22-25). These five eligible studies included 472 samples. All the studies measure Twist expression level by IHC. Three studies included stage I-IV lung cancer patients, one study included only the patients with stage I, and the rest one provided no detail data. The criteria to define Twist positive was not entirely uniform. The basic characteristics of the five eligible publications are listed in Table 1.
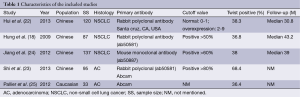
Full table
Meta-analysis results
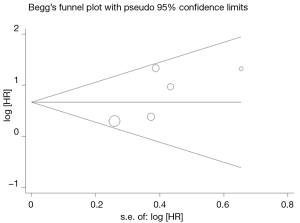
The combined HR for the five eligible studies was 1.949 (95% CI: 1.408-2.698, P<0.001), implying that Twist overexpression was an indicator of poor survival 0for NSCLC patients (Figure 1). Heterogeneity of overall prognosis was relatively low (I2=42.7%), as shown in Figure 2. We further undertook the subgroup analysis, the HR of Chinese patients was 1.864 (95% CI: 1.332-2.609) while the HR of Caucasian was 3.740 (95% CI: 1.040-13.500). When grouped by stage, the HR of overall stage I-V was 1.569 (95% CI: 1.079-2.283) while the HR for stage I was 3.780 (95% CI: 1.768-8.082). To determine the effect of an individual study on the summary meta-analysis estimate, we performed sensitivity analyses and no significant change was founded. Begg’s funnel plot and Egger’s test were performed to assess the publication bias in the literature. All five eligible studies yielded a Begg’s test score of P=0.221 and Egger’s test score of P=0.157. These results suggest that there is no publication bias (Figure 3).
Discussion
Twist is a highly conserved developmental gene that had been found to induce cancers promoting through EMT. Over the past decade, the reactivation of TWIST embryonic transcription factors has been described as a marker of poor prognosis in a large set of human cancers (2,26-28). However, the consensus about prognostic value of Twist in lung cancer has yet to reach. Our meta-analysis based on the results of Oncomine, which indicated the high expression of Twist in lung cancer. We then conduct meta-analysis analyzed the prognosis value of Twist specifically in lung cancer. And our results suggested that expression of Twist was associated with worse survival.
Based on our results, Twist might be a poor prognostic indicator for lung cancer. The subgroup analysis by population implied that the unfavorable prognosis value of twist could be detected in both Chinese and Caucasian patients. Although the pooled HR in Caucasian seemed to be higher, it could not demonstrate anything because of only one study conducted trial on Caucasian. In addition, few researches identified different prognostic value of Twist between populations. Therefore, more high quality studies are needed to further explore the prognosis value in different population.
It has been proved that biomarkers can be detected in cancerous tissue, blood, and body fluids. However, IHC is considered to be the best choice for examination of cancerous tissue samples. Therefore, we only included the studies that evaluated Twist by IHC. By the selection, it was consistency in the evaluation process among studies which may reduce the heterogeneity. Recently, Beck et al. (29) uncover a key role for Twist in the early steps of tumor initiation and a dose-dependent role in malignant progression. It suggested that different level of Twist could regulated the different carcinoma process, including tumor initiation, progression, propagation, and EMT by different mechanisms. The uniform evaluation method and appropriate cut-off value are important to identify the prognostic value of Twist accurately.
There are some limitations in this meta-analysis. Firstly, the sample size (SS) of our study was relatively small which is unfavorable to get powerful results. Secondly, we limited our analysis to studies which used IHC as detection method. However, the individual used different primary antibody and the antibody dilution, the method and evaluation of staining, which might affect the IHC sensibility. As previously mentioned, the uniform definition of high Twist expression is more helpful to obtain accurate results. Besides, the cut-off criteria and thresholds of cut-off value were subjective and not uniform in each article. Lastly, the expression of Twist in squamous cell cancer is likely to be highest according to Oncomine. However, we the primary article could not provide sufficient information for further analysis by histological subtypes.
Despite the limitations, our meta-analysis evaluated the prognostic value of Twist in lung cancer. We specially evaluated the risk of high Twist level to worse survival in lung cancer. And the heterogeneity in our results was relatively low, which implied the robustness of our results.
Conclusions
Based on our results, the Twist could be a poor prognostic indicator and is potential to be target for treating lung cancer. To substantiate our current results and further explore, adequate prospective studies are needed.
Acknowledgements
Funding: The study was supported by the National Natural Science Foundation of China (NO. 81302032).
Disclosure: The authors declare no conflict of interest.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [PubMed]
- Hosono S, Kajiyama H, Terauchi M, et al. Expression of Twist increases the risk for recurrence and for poor survival in epithelial ovarian carcinoma patients. Br J Cancer 2007;96:314-20. [PubMed]
- Burotto M, Thomas A, Subramaniam D, et al. Biomarkers in early-stage non-small-cell lung cancer: current concepts and future directions. J Thorac Oncol 2014;9:1609-17. [PubMed]
- D’Amico TA. Molecular biologic staging of lung cancer. Ann Thorac Surg 2008;85:S737-42. [PubMed]
- Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139:871-90. [PubMed]
- Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science 2013;342:1234850. [PubMed]
- Micalizzi DS, Ford HL. Epithelial-mesenchymal transition in development and cancer. Future Oncol 2009;5:1129-43. [PubMed]
- Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008;133:704-15. [PubMed]
- Kang Y, Massagué J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell 2004;118:277-9. [PubMed]
- Sánchez-Tilló E, Liu Y, de Barrios O, et al. EMT-activating transcription factors in cancer: beyond EMT and tumor invasiveness. Cell Mol Life Sci 2012;69:3429-56. [PubMed]
- Iwatsuki M, Mimori K, Yokobori T, et al. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci 2010;101:293-9. [PubMed]
- Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell 2006;127:679-95. [PubMed]
- Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol 2014;16:488-94. [PubMed]
- Lee TK, Poon RT, Yuen AP, et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res 2006;12:5369-76. [PubMed]
- Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004;117:927-39. [PubMed]
- Wushou A, Hou J, Zhao YJ, et al. Twist-1 up-regulation in carcinoma correlates to poor survival. Int J Mol Sci 2014;15:21621-30. [PubMed]
- Miura N, Yano T, Shoji F, et al. Clinicopathological significance of Sip1-associated epithelial mesenchymal transition in non-small cell lung cancer progression. Anticancer Res 2009;29:4099-106. [PubMed]
- Hung JJ, Yang MH, Hsu HS, et al. Prognostic significance of hypoxia-inducible factor-1alpha, TWIST1 and Snail expression in resectable non-small cell lung cancer. Thorax 2009;64:1082-9. [PubMed]
- Rhodes DR, Kalyana-Sundaram S, Mahavisno V, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia 2007;9:166-80. [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [PubMed]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [PubMed]
- Hui L, Zhang S, Dong X, et al. Prognostic significance of twist and N-cadherin expression in NSCLC. PLoS One 2013;8:e62171. [PubMed]
- Shi Y, Wu H, Zhang M, et al. Expression of the epithelial-mesenchymal transition-related proteins and their clinical significance in lung adenocarcinoma. Diagn Pathol 2013;8:89. [PubMed]
- Jiang W, Pang XG, Wang Q, et al. Prognostic role of Twist, Slug, and Foxc2 expression in stage I non-small-cell lung cancer after curative resection. Clin Lung Cancer 2012;13:280-7. [PubMed]
- Pallier K, Cessot A, Côté JF, et al. TWIST1 a new determinant of epithelial to mesenchymal transition in EGFR mutated lung adenocarcinoma. PLoS One 2012;7:e29954. [PubMed]
- Wushou A, Pan HY, Liu W, et al. Correlation of increased twist with lymph node metastasis in patients with oral squamous cell carcinoma. J Oral Maxillofac Surg 2012;70:1473-9. [PubMed]
- Kyo S, Sakaguchi J, Ohno S, et al. High Twist expression is involved in infiltrative endometrial cancer and affects patient survival. Hum Pathol 2006;37:431-8. [PubMed]
- Lee KW, Kim JH, Han S, et al. Twist1 is an independent prognostic factor of esophageal squamous cell carcinoma and associated with its epithelial-mesenchymal transition. Ann Surg Oncol 2012;19:326-35. [PubMed]
- Beck B, Lapouge G, Rorive S, et al. Different levels of Twist1 regulate skin tumor initiation, stemness, and progression. Cell Stem Cell 2015;16:67-79. [PubMed]