CHRNA3 rs1051730 polymorphism and lung cancer susceptibility in Asian population: a meta-analysis
Introduction
Lung cancer remains the deadliest cancer worldwide despite improvements in diagnostic and therapeutic techniques (1). Its incidence has yet to peak in many parts of world, particularly in China, which has become a major public health challenge (2). The mechanism of lung carcinogenesis is still not fully understood. Besides smoking status established as the most important single factor in causing lung cancer, host factors, including genetic polymorphisms, have been some growing interest in the study of the tumorigenesis of lung cancer (3). Many environmental carcinogens require metabolic activation by various drug-metabolizing enzymes.
The chromosome 15q25.1 region has been identified as a hotspot for lung cancer susceptibility by recent genome-wide association (GWA) studies (4,5). A single nucleotide polymorphism (SNP), rs1051730, maps to an 88-kb region on the long arm of chromosome 15q25.1. The predominant homozygous allele, the heterozygous allele and the homozygous rare allele of the CYP1A2 rs2069514 polymorphism are named the homozygous wild-type genotype (G/G), an allele carriers (G/A) and the rare homozygote (A/A), respectively.
The particular variant of rs1051730 has been investigated broadly in lung cancer and is shown to be significantly associated with ascending lung cancer risk especially among Caucasians (6-8). However, in Asians, the evidence of the association of rs1051730 polymorphism and lung cancer risk is insufficient. A single study may be too underpowered to detect a possible small effect of the polymorphisms on lung cancer, especially when the sample size is relatively small. Different types of study populations may also contribute the disparate findings. Hence, we performed a meta-analysis of all eligible studies to derive a more precise estimation of the associations of CHRNA3 rs1051730 polymorphism with lung cancer.
Materials and methods
Publication search
The electronic databases PubMed were searched for studies to include in the present meta-analysis, using the terms: “CHRNA3” or “rs1051730” or “polymorphism” and “lung cancer”. An upper date limit of Jan 01, 2015 was applied; no lower date limit was used. The search was performed without any restrictions on language and was focused on studies that had been conducted in humans. Concurrently, the reference lists of reviews and retrieved articles were searched manually. Only full-text articles were included. When the same patient population appeared in several publications, only the most recent or completes study was included in this meta-analysis.
Inclusion criteria
The included studies have to meet the following criteria: (I) evaluating the CHRNA3 rs1051730 polymorphism and lung cancer risk in Asian population; (II) case-control studies; and (III) supply the number of individual genotypes for CHRNA3 rs1051730 genotype in lung cancer cases and controls, respectively.
Data extraction
Information was carefully extracted from all eligible publications independently by two authors according to the inclusion criteria listed above. Disagreement was resolved by discussion between the two authors.
The following data were collected from each study: first author’s surname, year of publication, ethnicity, total numbers of cases and controls, and numbers of cases and controls with the CC, CT and TT genotypes, respectively. If data from any of the above categories were not reported in the primary study, items were treated as “not applicable”. We did not contact the author of the primary study to request the information. We did not require a minimum number of patients for a study to be included in our meta-analysis.
Statistical analysis
Odds ratio (OR) with 95% confidence interval (CI) was used to assess the strength of association between the CHRNA3 rs1051730 polymorphism and lung cancer risk. The pooled ORs for the risk associated with the genotypes of homozygote C/C and T allele carriers (C/T + T/T) with the C/C genotype were calculated. Subgroup analyses were done by ethnicity. Heterogeneity assumption was checked by the chi-square-based Q-test (9). A P value greater than 0.10 for the Q-test indicates a lack of heterogeneity among studies, so the pooled OR estimate of the each study was calculated by the fixed-effects model (the Mantel-Haenszel method) (10). Otherwise, the random-effects model (the DerSimonian and Laird method) was used (11). One-way sensitivity analyses were performed to assess the stability of the results, namely, a single study in the meta-analysis was deleted each time to reflect the influence of the individual data-set to the pooled OR (12). An estimate of potential publication bias was carried out by the funnel plot, in which the standard error of log (OR) of each study was plotted against its log (OR). An asymmetric plot suggests a possible publication bias. Funnel plot asymmetry was assessed by the method of Egger’s linear regression test, a linear regression approach to measure the funnel plot asymmetry on the natural logarithm scale of the OR. The significance of the intercept was determined by the t-test suggested by Egger (P<0.05 was considered representative of statistically significant publication bias) (13). All of the calculations were performed using Stata version 11.0 (Stata Corporation, College Station, TX, USA).
Results
Study characteristics
A total of four publications involving 2,890 lung cancer cases and 2,521 controls met the inclusion criteria and were ultimately analyzed (14-17). Table 1 presents the main characteristics of these studies. Among the four publications, all were published in English. The sample sizes ranged from 410 to 2,117. Almost all of the cases were histologically confirmed. Controls were mainly healthy populations. There were two groups of Chinese, two groups of Japanese population.

Full table
Meta-analysis results
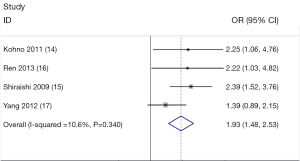
Overall, for the T allele carriers (C/T + T/T) and the homozygote T/T, the pooled ORs for all studies combined 2,890 cases and 2,521 controls were 1.93 (95% CI =1.48-2.53, P=0.34 for heterogeneity) and 1.63 (95% CI =1.27-1.99, P=0.46 for heterogeneity) (Figure 1), when compared with the homozygous wild-type genotype (C/C).
Sensitivity analyses
A single study involved in the meta-analysis was deleted each time to reflect the influence of the individual data set to the pooled ORs, and the corresponding pooled ORs were not materially altered (data not shown).
Publication bias
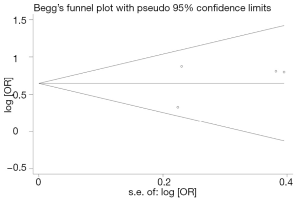
Begg’s funnel plot and Egger’s test were performed to access the publication bias of literatures. Evaluation of publication bias for T allele carriers (C/T + T/T) versus CC showed that the Egger test was not significant (P=0.372). The funnel plots for publication bias (Figure 2) also did not show some asymmetry. These results did not indicate a potential for publication bias.
Discussion
To we knowledge, this meta-analysis is firstly available for comprehensively evaluating the associations between CHRNA3 rs1051730 polymorphism and lung cancer risk in Asian population. This meta-analysis summarize all the available data on the association between CHRNA3 rs1051730 polymorphism and lung cancer risk, including a total of 2,890 lung cancer cases and 2521 controls. Our results indicated that CHRNA3 rs1051730 polymorphism contributes to risk of lung cancer among Asian population.
Recently, Ren et al. (16) conducted a case-control study comprising of 210 histologically confirmed lung cancer cases and 200 healthy controls to examine CHRNA3 rs1051730 and lung cancer risk, in a Chinese Han population. They found that rs1051730 polymorphism may modify susceptibility to lung cancer via a smoking-independent manner, which is consistent with our results of this meta-analysis. However, another two compressive studies suggested that rs1051730 might not be a risk-conferring factor for lung cancer in East-Asians (18,19). The inconsistent results may be partly due to selection bias, partly due to inconformity to Hardy-Weinberg equilibrium in some cases and partly due to different linkage disequilibrium patterns in different population.
Some limitations of this meta-analysis should be acknowledged. Firstly, heterogeneity is a potential problem when interpreting all the results of meta-analyses. Although we minimized the likelihood by performing a careful search for published studies, using the explicit criteria for study inclusion, performing data extraction and data analysis strictly, the significant between-study heterogeneity still existed in almost each comparison. The presence of heterogeneity can result from differences in the selection of controls, age distribution, the lifestyle factors and so on. Although most of the controls were selected from healthy populations, some studies had selected controls among friends or family of lung cancer patients or patients with other diseases. Secondly, only published studies were included in this meta-analysis. The presence of publication bias indicates that non-significant or negative findings may be unpublished. Lastly, our results were based on unadjusted estimates, while a more precise analysis should be conducted if individual data were available, which would allow for the adjustment by other covariates including age, ethnicity, family history, environmental factors and lifestyle.
In conclusion, this meta-analysis suggests that the CHRNA3 rs1051730 polymorphism is associated with lung cancer risk among Asian population. In addition, it is necessary to conduct large trials using standardized unbiased methods, homogeneous lung cancer patients and well matched controls, with the assessors blinded to the data.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [PubMed]
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [PubMed]
- Toh CK, Gao F, Lim WT, et al. Never-smokers with lung cancer: epidemiologic evidence of a distinct disease entity. J Clin Oncol 2006;24:2245-51. [PubMed]
- Amos CI, Wu X, Broderick P, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet 2008;40:616-22. [PubMed]
- Thorgeirsson TE, Geller F, Sulem P, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 2008;452:638-42. [PubMed]
- Amos CI, Wu X, Broderick P, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet 2008;40:616-22. [PubMed]
- Hung RJ, McKay JD, Gaborieau V, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature 2008;452:633-7. [PubMed]
- Thorgeirsson TE, Geller F, Sulem P, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 2008;452:638-42. [PubMed]
- Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101-29.
- Mantel N, Haenszel W.. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719-48. [PubMed]
- DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [PubMed]
- Bhat IA, Pandith AA, Bhat BA, et al. Lack of association of a common polymorphism in the 3' -UTR of interleukin 8 with non small cell lung cancer in Kashmir. Asian Pac J Cancer Prev 2013;14:4403-8. [PubMed]
- Kohno T, Kunitoh H, Mimaki S, et al. Contribution of the TP53, OGG1, CHRNA3, and HLA-DQA1 genes to the risk for lung squamous cell carcinoma. J Thorac Oncol 2011;6:813-7. [PubMed]
- Shiraishi K, Kohno T, Kunitoh H, et al. Contribution of nicotine acetylcholine receptor polymorphisms to lung cancer risk in a smoking-independent manner in the Japanese. Carcinogenesis 2009;30:65-70. [PubMed]
- Ren JH, Jin M, He WS, et al. Association between CHRNA3 rs1051730 genotype and lung cancer risk in Chinese Han population: a case-control study. J Huazhong Univ Sci Technolog Med Sci 2013;33:897-901. [PubMed]
- Yang L, Qiu F, Lu X, et al. Functional polymorphisms of CHRNA3 predict risks of chronic obstructive pulmonary disease and lung cancer in Chinese. PLoS One 2012;7:e46071. [PubMed]
- Sinha P, Logan HL, Mendenhall WM. Human papillomavirus, smoking, and head and neck cancer. Am J Otolaryngol 2012;33:130-6. [PubMed]
- Wu C, Hu Z, Yu D, et al. Genetic variants on chromosome 15q25 associated with lung cancer risk in Chinese populations. Cancer Res 2009;69:5065-72. [PubMed]


