SBRT in operable early stage lung cancer patients
Background
Malignant neoplasm of the lung is the most frequent cause of cancer related death in males and second in females.
Early stage non-small cell lung cancer (NSCLC) is cured in many cases by local treatment. Unfortunately three quarters of NSCLC cases are detected in a later stage of disease due to a lack of clinical symptoms. Cure is then only achieved in few patients. However, the combination of population aging and oncoming CT-based screenings programs will increase the number of diagnosed early stage lung cancer especially in the elderly patients (1,2).
In the past, surgical lobectomy plus mediastinal lymph node dissection was established as the standard treatment in operable patients. Patients with higher surgical risk due to comorbidity may undergo sublobar resection, although its outcome is inferior based on a randomized study (3). About 80% of stage I disease patients undergo surgical resection (4). However in treatment of elderly patients with increasing numbers of comorbidities, the value of surgery will decrease (5). In the USA the percentage of patients with age >85 years as well as having >3 comorbidities doubled between 1998 and 2007. The number of patients treated with no local therapy at all increased from 14.6% in 1998 to 18.3% in 2007. Looking at these data the decline in use of surgical resection from 75.2% to 67.3%, despite the increasing use of less invasive (6) video-assisted thoracoscopic surgery (VATS), isn’t surprising (7). According to data from the Netherlands this proportion even drops <40% in patients >75 years (8). Best supportive care without curative treatment intention is practiced with increasing frequency. Vest et al. report of a growing proportion not receiving a curative local treatment from 14.6% in 1998 to 18.3% in 2007 in the USA (7). This number increases in patients >75 years up to 26% (9). Five-year cancer-specific-survival is about 14% (10) in patients undergoing best supportive care indicating the need for a curative and simultaneously minimally or non-invasive treatment option.
For inoperable patients so-called conventional radiation treatment is an established curative treatment option. Conventional radiation in this context usually means applying 60-66 Gy in 2 Gy-fractions over a time period of 6-7 weeks. Overall survival (OS) of about 30% and cancer specific survival (CSS) of about 50% after 3 years can be achieved in these non-operable patient cohorts (11). However, retrospective studies showed local tumor relapse being the most frequent site of treatment failure and proofed a correlation of dose escalation and OS (12-15).
During the last years improved results were achieved in non-operable patients by introduction of novel radiotherapy concepts and technologies: stereotactic-body-radiotherapy (SBRT). SBRT combines several modern technologies to accurately treat tumors with very high irradiation doses. These irradiation doses are delivered in few radiotherapy fractions or even in one radiosurgical session. Safety of this radical but non-invasive treatment is achieved by confinement of high irradiation doses to the tumor and sparing of healthy normal tissue.
History
SBRT evolved from cranial stereotactic radiotherapy (SRT) by transferring its principles and practice to extracranial sites. Pioneer work done in the mid-1990s at the Karolinska Hospital in Sweden and this concept was quickly adopted and further developed in Japan and Germany (16-19).
Stereotaxy started out as a form of neurosurgery that uses a mechanical head frame and a precise 3-dimensional (3D) coordinate system to align and direct surgical instruments. This combination of a rigid frame and a constitutive 3D-coordinate system was used in radio-oncology for better patient-fixation and treatment planning. With improvement and development of modern imaging systems the coordinates could be referred to the imaging data-sets and non-invasive fixation systems replaced rigid frames. This opened the path for stereotactic radiation therapy to target extracranial tumor sites.
Definition of SBRT
Several work groups have given their version of a definition of SBRT (20-24). A consensus can be described as followed: SBRT is a method of external beam radiotherapy (EBRT) that accurately delivers a high dose of irradiation in one or few treatment fractions to an image-defined extracranial target. Shifting from conventional RT to SBRT is not only a simple modification of techniques, but should be considered as a complete replacement of concepts. More precise methods in terms of localizing and tracking the tumor, fixation of the patient, planning techniques and application of radiotherapy itself, are needed to apply hypo-fractionated doses as used in SBRT. However, by applying the SBRT-concept the whole diagnosis and treatment work flow and not only technical issues have to be adapted (20).
Clinical outcome of SBRT
SBRT in non-operable patients
Conventional radiation therapy has been proven to provide better outcome than best supportive care (25) and was therefore considered to be the first-line therapy in non-operable early stage lung cancer patients. Some years ago this changed in favor of SBRT. NCCN Guidelines as well as the ESMO Clinical Practice Guidelines consider SBRT as first line treatment in medically inoperable patients (26,27).
It’s an attractive treatment option for several reasons: non-invasive, outpatient-basis and short overall treatment time of 1-2 weeks.
Compared to best supportive care
Population-based analyses from the Netherlands (8,28) and the US (29) demonstrated an improvement in OS for stage I NSCLC in elderly patients by introducing SBRT.
Haasbeek showed that OS improved in patients treated with radiotherapy by introducing SBRT from 16 months to 24 months between 2001 and 2009 in the Netherlands (8). Palma et al. showed a corresponding increase from 16 months to 21 months in elderly patients in North Holland, regardless of treatment modality (28). Furthermore both showed that availability of SBRT reduced the proportion of patients receiving non-curative treatment by 7-12%. Simultaneously, the proportion of patients that underwent surgery remained constant.
The US study is based on the SEER database of patients older than 65 years and compared five different treatment options for patients with stage I NSCLC (29): best supportive care, conventional radiotherapy, SBRT, sublobar resection and lobectomy. Propensity score matching between SBRT and non-SBRT treatment was performed to correct for imbalances of race, sex, education level, median income, comorbidity score, histology, tumor grade, tumor size, and presence of lymph node sampling. SBRT achieved improved OS compared to best supportive care and conventional radiotherapy and differences were not significant compared to sublobar resection and lobectomy.
Compared to conventionally fractionated radiotherapy
Several prospective phase II trials have been conducted and 2-3 years local tumor control and OS ranged between 84-98% and 43-72%, respectively.
Prospective trials (see Table 1) showed 2-3 years local tumor-control rates of 84-98% and OS between 43-72% in non-operable patients suffering from early-stage NSCLC and treated with SBRT (24,35-37,41,42). Even though different SBRT methodologies were used the results were similar and highly consistent.
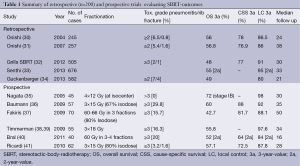
Full table
As better local tumor control was shown to go along with higher OS in patients treated with conventional radiation therapy (12-15), it can also be shown that even further improvement of local control (LC) by applying SBRT transfers into even better OS (29). In a meta-analysis done by Grutters et al., 2-year OS for SBRT was 70% vs. 53% for CRT and 2-year CSS was 83% vs. 67% (43).
Large retrospective analyses confirmed the good results described above in clinical practice outside of prospective clinical trials. Only studies with >200 patients are included in Table 1, which summarizes a total of 2,265 cases. The outcome of 582 patients treated at 13 German and Austrian centers was analyzed (34): it was shown that local tumor control and OS were independent from SBRT-technology used at different time periods and at different centers. Furthermore dose escalation was again shown as a significant factor influencing OS and LC. A biological effective dose BED of at least 106 Gy (2 Gy equivalent) resulted in a 3-year LC rate of 92.5% compared to 79.6% in all patients. three-year OS increased from 47.1% to 62.2%. This dose dependency of local failure was also seen by Onishi et al. They reported a cut-off-value at a BED =100 Gy leading to a 3-year OS of 88.9% compared with 69.4% in medically operable patients (30,31). The data collected by Grills et al. showed a better tumor control in patients treated with more than a BED of 105 Gy (32). A meta-analysis done by Zhang shows that the outcome gets worse for a BED below 83.2 Gy and a BED that exceeds 146 Gy. Therefore the favorable dose should be in between (44). OS is mainly affected by distant metastases and comorbidities. The probability of distant metastases is up to 20-26% of cases and is correlated to lesion size (33,38,45,46).
Numerous pro- and retrospective studies have confirmed good SBRT results. High consistency between the studies and reproducibility of results in clinical daily routine even in change of clinical setting can be seen. This is a strong indicator for quality and robustness of SBRT treatment.
Compared to radiofrequency ablation (RFA)
RFA alone (47) or in combination with conventional radiotherapy (48) has been introduced as a minimally invasive option into the treatment of stage I NSCLC. No study performed a direct comparison between SBRT and RFA but a recent literature review reported improved local tumor control, CSS and OS after SBRT compared to RFA (49). Additionally, toxicity and 30-day mortality (50) were lower after SBRT resulting in the conclusion, that SBRT should be proposed as the first non-surgical treatment to high-risk patients.
SBRT in medically operable patients compared to surgery
First-line treatment in operable stage I NSCLC patients is surgery: lobectomy proved to achieve better outcome than wedge resection (51). Today sublobar anatomical resection (segmentectomy) is discussed as another option (52,53); whether segmentectomy delivers worse (3) or comparable outcome compared to lobectomy is still under investigation (54,55).
Based on the highly promising outcome of SBRT in medically inoperable patients, three randomized trials comparing SBRT with lobectomy (ROSEL, STAR) or sublobar resection (ACOSOG Z4099/RTOG 1021) (56) have been started but all three studies closed very early due to poor accrual: <5% of the planned patients were enrolled leaving us without level A evidence.
Hence level A evidence won’t be available in the near future. Several studies compared SBRT to surgery using statistical methods like matched pair analyses and propensity score matching to correct for imbalances in patient characteristics.
Grills et al. performed a retrospective single-institution comparison between SBRT and wedge resection. Improved local tumor control in favor of SBRT (5% vs. 24%) with no differences in CSS was reported. OS was better in the surgical cohort, which was explained by older age and increased comorbidities in the SBRT patients (57). The previously cited US population based SEER analysis showed no difference in OS and CSS for SBRT versus sublobar resection or lobectomy after propensity score matching (29). Moreover SBRT was shown to be the treatment with best OS up to 6 months in the total of patients, showing its superiority in morbidity and treatment-related mortality.
Puri et al. reported identical CSS between SBRT and surgery (lobectomy in 80% of the patients) (58). OS appeared better after surgery compared to SBRT but was not statistically significant and this potential difference was explained by increased pulmonary comorbidities in the SBRT cohort, which was not corrected in the propensity score matching. Verstegen et al. compared SBRT and VATS lobectomy in 128 patients after propensity score matching of gender, age, clinical tumor stage, tumor diameter, location of the tumor, pretreatment tumor histology, lung function (FEV1%), Charlson comorbidity score and WHO performance score. Locoregional control was better after SBRT with no differences in freedom from progression and OS (59). A total of 257 propensity scored patients were analyzed by Crabtree et al. and there was again no difference seen between local recurrence, CSS or OS after 3 years (60).
Few studies reported outcome after SBRT when patients were considered suitable for surgical resection but surgery was actively refused by the patients. Two Japanese and one Dutch study described excellent OS of 70% after 5 years (n=87) (61), 86% after 3 years (n=29) (62) and 85% at 3 years (n=177) (63), respectively, results which compare well to OS after lobectomy. Uematsu reported a 3-year OS of 86% in medically operable patients (62). A Markov Model-based decision analysis was developed by Louie et al. comparing SBRT and lobectomy. They postulated a comparable OS and quality-adjusted life expectancy (64).
Palma et al. reported of comparable outcome in COPD patients undergoing surgical resection or SBRT. However 30-day mortality was significantly higher (0% vs. 10%) in surgical patients (65). This compares with a low 30-day mortality rate after SBRT in general (34). Grills et al. described no treatment-related death in a nonrandomized retrospective analysis comparing wedge resection with SBRT. Nevertheless a higher 30-day readmission rate in the wedge resection group was conspicuous (57).
Consequently, SBRT appears as a viable treatment option in the situation, when lobectomy is refused by the patients. Additionally, SBRT appears equivalent to sublobar resection and both options with their specific pros and cons should be discussed with the patient.
Toxicity and quality of live after lung SBRT
The majority of patients treated with SBRT suffer from severe pulmonary or cardiovascular comorbidities and their poor pulmonary status, which does not allow surgical resection. Consequently pulmonary toxicity is an important point of concern in lung SBRT. Radiation induced pneumonitis (RP) is usually seen after a median of 5 months which is longer compared to conventional radiotherapy (66). The treatment of peripherally located tumors <5 cm in diameter causes RP in below 10% of cases. Risk of RP is reported to be dependent on planned target volume (PTV), mean lung dose and low-dose spread for conventional radiotherapy (67,68). The conclusion that risk factors are similar in SBRT is supported by several papers (66,69-73). RP grade ≥II ranges from below 10% in the majority of reports up to even 28% in one report (66,69,72-80). Development of high grade RP after stereotactic treatment is rarely reported. The two largest retrospective papers show an incidence of RP Tox. Grade ≥2 of below 8% (32,34). Patients with pre-existent pulmonary fibrosis might be at increased risk for RP.
Additionally, pulmonary function is stable after SBRT with a loss of <10% (FEV1, DLCO) within 24 months after treatment (81,82). Pulmonary toxicity was not increased even in patients with very poor pre-SBRT pulmonary function and with severe COPD GOLD III-IV (82). Bishawi et al. even postulated a better pulmonary function after four months from SBRT for non-COPD-patients because of tumor shrinkage (83).
Chest wall toxicity (myositis, neuralgia, rip fracture, subcutaneous fibrosis, and skin ulceration) has been reported when tumors are located close to the respective normal tissue structures. Doses >30 Gy (delivered in 3 fractions) to the chest wall haven been correlated with these toxicities and the volume of the chest wall exposed to these doses should be minimized by conformal treatment planning (61,84-90). Based on their data, Mutter et al. suggest a 30 Gy constraint to a max of 70 cm3 of the chest wall (2 cm expansion of the lung) to prevent chest wall pain.
Severe toxicity to the brachial plexus (neuropathic pain, motor weakness, or sensory alteration), large bronchi (stenosis with pulmonary atelectasis) and esophagus (ulceration, perforation, fistula) has been reported but these toxicities are rare. Limiting the total dose to the plexus to <26 Gy in 3-4 fractions can minimize the risk of toxicity (91).
Whereas safety of such high single and total doses has been demonstrated for peripheral lung tumors of usually <5 cm size, higher rates of severe toxicity have been reported in centrally located tumors with critical organs like the esophagus and large bronchi close by (92,93). Occurrence of these toxicities is known from conventional radiotherapy to centrally located tumors and therefore not unforeseen (94).
Some reports even mention treatment-related deaths, especially in centrally located tumors (40,95). Senthi et al. reported of a treatment-related death rate of up to 2.7%, respectively of 1% if BED below 210 Gy is used. In contrast, safety of SBRT for centrally located tumors has been reported if the total dose is delivered using a larger number (5-10) of treatment fractions and a lower single-fraction dose (33). Considerable volume definition and avoidance of multiple treatments to the same hilar bronchus is recommended (96) in order to prevent central toxicities like major airway occlusion (97).
Studies consistently reported that SBRT has no detrimental or negative on quality-of-life (QoL) (98-100). Overall QoL as well as subdomains of dyspnea and cough were stable after SBRT in all studies and one study described significantly improved emotional functioning (98).
Clinical implemention of SBRT for early stage NSCLC
Before technical details of SBRT will be discussed, it is of fundamental importance that SBRT is practiced by a dedicated multidisciplinary team. All members of this team—radiation oncologists, medical physicists and radiation technologists—should receive specific training and gain experience in SBRT and treatment needs to follow written guidelines.
Several groups and organizations published their recommendation to best practice of SBRT and a short summary is given below.
Clinical evaluation
Evaluation of performance status and pulmonary function is necessary to enable a sensible treatment concept. In surgical series, higher perioperative morbidity and lower quality of life is correlated to higher age (>70 years) and the presence of other comorbidities (5,101,102). To get an impression of the patients risk to suffer from treatment-complications, pulmonary function testing like maximal oxygen uptake (VO2max), forced expiratory volume in 1 second (FEV1) or diffusion capacity (DCO) is essential for both postoperative and post-radiation performance (102,103). Worse performance status and FEV1 were proven to correlate with higher side effects in normo-fractioned radiation therapy (104).
Histo-pathological confirmation of lung cancer
Whenever possible and reasonable a biopsy for histopathological confirmation of the cancer diagnosis should be performed. However transbronchial biopsy or transthoracic fine needle aspiration is sometimes impossible due to unacceptable risks or may fail to prove malignancy.
In this case clinical (age, smoking habit, history of prior malignancy) and radiological criteria (diameter, spiculation, nodule growth rate) are proven to be good prediction or risk factors for malignancy (105-112). The volume doubling time of malignant nodules is somewhere between 20-400 and most often around 120 days (113,114). Nodules, that grow faster or slower have a higher probability to be benign (111). In addition a PET-CT scan might help to evaluate the probability, as higher glucose metabolism is an indicator for malignancy (115).
Repeated imaging to evaluate the growth pattern is an option in patient with intermediate risk of malignancy. However, observation might put the patient at risk of disease progression (116). Although probability of tumor cell dissemination rises with stage of disease, even small primary pulmonary lesions are able to cause disseminated disease (117-120). Therefore the point of time when curative local treatment has the possibility to be successful might be missed.
If malignancy is highly likely based on the described criteria, immediate SBRT without histopathological confirmation is justified (121), as is in this population also standard practice in thoracic surgery (29,122).
As SBRT is also a way of curative treatment of unfit patients that would otherwise have gone to best supportive care, the percentage of histopathological confirmation is already decreasing as the risk for invasive confirmation might be too high (9).
Staging of disease
Correct disease staging is essential for treatment indication because only the primary tumor without elective nodal irradiation is treated in SBRT. Several working groups have given their recommendations referring to staging procedures prior to SBRT (20,21).
Chest-CT-scan using intravenous contrast including the upper abdomen is mandatory.
A whole body FDG-PET/CT-scan might not only improve the malignancy prediction model as mentioned earlier, but there’s also evidence of increased detection accuracy of nodal and/or distant metastases (123-125). Even though this is still a subject of discussion for early stage lung cancer (126,127). A FDG-PET/CT scan as part of disease staging is widely postulated (20,21). Furthermore, a PET-CT scan serves to exclude clinically relevant distant metastases or second malignancies.
Pathological FDG uptake in mediastinal lymph nodes should lead to histopathological evaluation in order to prevent overstating (127). Endoscopic (EUS) or endobronchial ultrasound (EBUS) can be used for biopsy guidance. If the situation is still unclear, a mediastinoscopy may be necessary.
Interdisciplinary decision making
SBRT is a local modality that complements other surgical and non-surgical treatments.
As a corollary of this and the big efforts that are made to lay the foundation for high quality treatment, indication for SBRT should be discussed in a multidisciplinary tumor board to offer the patient a therapy concept, that’s sensible, individualized and which ensures a high level of quality.
Treatment planning
Imaging for target volume and organ at risk (OAR) definition is a key factor for successful SBRT practice. Only macroscopic targets and small, immediately adjacent volumes of potential microscopic spread are treated in SBRT. 4D-imaging is essential to evaluated breathing induced tumor motion on a patient individual basis. Breathing induced target motion requires motion management strategies to minimize the dose delivered to non-pathological tissue. Several different approaches can be applied and have already been implemented into routine practice (128). In principle, we distinguish between passive 4D motion management strategies and active strategies, where treatment is adapted in real-time to breathing motion. Despite huge technical differences between the strategies, no difference in clinical outcome has been reported.
A minimum dose of at least 100 Gy BED in 3-8 fractions is mandatory as described above. In this context the importance of reassuring the delivery of the prescribed dose was shown by Latifi et al. They report of a higher recurrence rate for patients planned with Pencil-Beam compared to collapsed cone convolution (CCC)-algorithm even though the prescribed nominal dose and constraints were identical. This has been conducted to a relative dosimetric underdosing (129).
Patient immobilization and setup
Accurate target localization is essential to apply the conformal radiation dose to the target volume and to spare critical organs at risk. Strict immobilization by patient-customized systems enable reproducible patient setup and reduce inter- and intrafraction motion of the patients’ bony anatomy. To reduce uncertainties to a minimum, daily pretreatment imaging is an essential part of each and every treatment session.
Breathing induced target motion, setup-errors and base-line shifts must be taken into account. Image guidance can be achieved through both: visualization of the lung tumor directly or implanted fiducial markers that act as a surrogate for tumor position. Post- and/or mid-treatment imaging is recommended for quality assurance, particularly in single fraction SBRT.
Follow-up
To confirm and validate efficacy, outcomes and toxicities after SBRT, early and late effects have to be assiduous documented. Special attention has to be brought to potential complications. Differentiation between post-SBRT fibrosis and local recurrence of disease is sometimes difficult. Huang et al. published a systematic literature review and proposed an algorithm for this important clinical issue (130). Because of these difficulties, clinical and radiological follow-up should therefore be performed at the treating institution, where all detailed information about the SBRT treatment is available.
Summary
SBRT is an evidence-based and effective treatment option for patients with stage I NSCLC. Superiority to best supportive care and conventional radiotherapy has been documented in prospective and retrospective studies. Local tumor control rates exceeding 90% is consistently achieved and OS is mainly limited by comorbidities. Equivalence to surgery has been consistently reported in matched pair analysis and studies using propensity score matching but level A evidence is missing due to a lack of successfully completed randomized trials: a multi-professional team experienced and trained in SBRT and image guided radiotherapy is essential for safe practice. Discussion in multidisciplinary tumor boards considering the perioperative risk of the patient and patient’s preference is important.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758-65. [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [PubMed]
- Whitson BA, Groth SS, Andrade RS, et al. Survival after lobectomy versus segmentectomy for stage I non-small cell lung cancer: a population-based analysis. Ann Thorac Surg 2011;92:1943-50. [PubMed]
- Raz DJ, Zell JA, Ou SH, et al. Natural history of stage I non-small cell lung cancer: implications for early detection. Chest 2007;132:193-9. [PubMed]
- de Perrot M, Licker M, Reymond MA, et al. Influence of age on operative mortality and long-term survival after lung resection for bronchogenic carcinoma. Eur Respir J 1999;14:419-22. [PubMed]
- Port JL, Mirza FM, Lee PC, et al. Lobectomy in octogenarians with non-small cell lung cancer: ramifications of increasing life expectancy and the benefits of minimally invasive surgery. Ann Thorac Surg 2011;92:1951-7. [PubMed]
- Vest MT, Herrin J, Soulos PR, et al. Use of new treatment modalities for non-small cell lung cancer care in the Medicare population. Chest 2013;143:429-35. [PubMed]
- Haasbeek CJ, Palma D, Visser O, et al. Early-stage lung cancer in elderly patients: a population-based study of changes in treatment patterns and survival in the Netherlands. Ann Oncol 2012;23:2743-7. [PubMed]
- Palma D, Visser O, Lagerwaard FJ, et al. Treatment of stage I NSCLC in elderly patients: a population-based matched-pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol 2011;101:240-4. [PubMed]
- Detterbeck FC, Gibson CJ. Turning gray: the natural history of lung cancer over time. J Thorac Oncol 2008;3:781-92. [PubMed]
- Chadha AS, Ganti AK, Sohi JS, et al. Survival in untreated early stage non-small cell lung cancer. Anticancer Res 2005;25:3517-20. [PubMed]
- Rowell NP, Williams CJ. Radical radiotherapy for stage I/II non-small cell lung cancer in patients not sufficiently fit for or declining surgery (medically inoperable): a systematic review. Thorax 2001;56:628-38. [PubMed]
- Martel MK, Ten Haken RK, Hazuka MB, et al. Estimation of tumor control probability model parameters from 3-D dose distributions of non-small cell lung cancer patients. Lung Cancer 1999;24:31-7. [PubMed]
- Partridge M, Ramos M, Sardaro A, et al. Dose escalation for non-small cell lung cancer: analysis and modelling of published literature. Radiother Oncol 2011;99:6-11. [PubMed]
- Sibley GS, Jamieson TA, Marks LB, et al. Radiotherapy alone for medically inoperable stage I non-small-cell lung cancer: the Duke experience. Int J Radiat Oncol Biol Phys 1998;40:149-54. [PubMed]
- Wulf J, Hädinger U, Oppitz U, et al. Stereotactic radiotherapy of extracranial targets: CT-simulation and accuracy of treatment in the stereotactic body frame. Radiother Oncol 2000;57:225-36. [PubMed]
- Uematsu M, Shioda A, Tahara K, et al. Focal, high dose, and fractionated modified stereotactic radiation therapy for lung carcinoma patients: a preliminary experience. Cancer 1998;82:1062-70. [PubMed]
- Lax I, Blomgren H, Näslund I, et al. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol 1994;33:677-83. [PubMed]
- Herfarth KK, Debus J, Lohr F, et al. Extracranial stereotactic radiation therapy: set-up accuracy of patients treated for liver metastases. Int J Radiat Oncol Biol Phys 2000;46:329-35. [PubMed]
- Guckenberger M, Andratschke N, Alheit H, et al. Definition of stereotactic body radiotherapy: principles and practice for the treatment of stage I non-small cell lung cancer. Strahlenther Onkol 2014;190:26-33. [PubMed]
- Potters L, Kavanagh B, Galvin JM, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2010;76:326-32. [PubMed]
- Kirkbride P, Cooper T. Stereotactic body radiotherapy. Guidelines for commissioners, providers and clinicians: a national report. Clin Oncol (R Coll Radiol) 2011;23:163-4. [PubMed]
- Sahgal A, Roberge D, Schellenberg D, et al. The Canadian Association of Radiation Oncology scope of practice guidelines for lung, liver and spine stereotactic body radiotherapy. Clin Oncol (R Coll Radiol) 2012;24:629-39. [PubMed]
- Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 2010;37:4078-101. [PubMed]
- Wisnivesky JP, Halm E, Bonomi M, et al. Effectiveness of radiation therapy for elderly patients with unresected stage I and II non-small cell lung cancer. Am J Respir Crit Care Med 2010;181:264-9. [PubMed]
- NCCN Clinical Practice Guidelines in Oncology: Non-small cell lung cancer Version 1.2013 [Internet]. Available online: http://www.nccn.org/
- Vansteenkiste J, De Ruysscher D, Eberhardt WE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi89-98. [PubMed]
- Palma D, Visser O, Lagerwaard FJ, et al. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol 2010;28:5153-9. [PubMed]
- Shirvani SM, Jiang J, Chang JY, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys 2012;84:1060-70. [PubMed]
- Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004;101:1623-31. [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. [PubMed]
- Grills IS, Hope AJ, Guckenberger M, et al. A collaborative analysis of stereotactic lung radiotherapy outcomes for early-stage non-small-cell lung cancer using daily online cone-beam computed tomography image-guided radiotherapy. J Thorac Oncol 2012;7:1382-93. [PubMed]
- Senthi S, Haasbeek CJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for central lung tumours: a systematic review. Radiother Oncol 2013;106:276-82. [PubMed]
- Guckenberger M, Allgäuer M, Appold S, et al. Safety and efficacy of stereotactic body radiotherapy for stage 1 non-small-cell lung cancer in routine clinical practice: a patterns-of-care and outcome analysis. J Thorac Oncol 2013;8:1050-8. [PubMed]
- Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2005;63:1427-31. [PubMed]
- Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290-6. [PubMed]
- Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009;75:677-82. [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [PubMed]
- Timmerman RD, Paulus R, Galvin J, et al. Stereotactic Body Radiation Therapy for Medically Inoperable Early-stage Lung Cancer Patients: Analysis of RTOG 0236. IJROBP 2009;75:S3.
- Bral S, Gevaert T, Linthout N, et al. Prospective, risk-adapted strategy of stereotactic body radiotherapy for early-stage non-small-cell lung cancer: results of a Phase II trial. Int J Radiat Oncol Biol Phys 2011;80:1343-9. [PubMed]
- Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung Cancer 2010;68:72-7. [PubMed]
- Pennathur A, Luketich JD, Heron DE, et al. Stereotactic radiosurgery for the treatment of stage I non-small cell lung cancer in high-risk patients. J Thorac Cardiovasc Surg 2009;137:597-604. [PubMed]
- Grutters JP, Kessels AG, Pijls-Johannesma M, et al. Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: a meta-analysis. Radiother Oncol 2010;95:32-40. [PubMed]
- Zhang J, Yang F, Li B, et al. Which is the optimal biologically effective dose of stereotactic body radiotherapy for Stage I non-small-cell lung cancer? A meta-analysis. Int J Radiat Oncol Biol Phys 2011;81:e305-16. [PubMed]
- Nath SK, Sandhu AP, Kim D, et al. Locoregional and distant failure following image-guided stereotactic body radiation for early-stage primary lung cancer. Radiother Oncol 2011;99:12-7. [PubMed]
- Andratschke N, Zimmermann F, Boehm E, et al. Stereotactic radiotherapy of histologically proven inoperable stage I non-small cell lung cancer: patterns of failure. Radiother Oncol 2011;101:245-9. [PubMed]
- Lanuti M, Sharma A, Willers H, et al. Radiofrequency ablation for stage I non-small cell lung cancer: management of locoregional recurrence. Ann Thorac Surg 2012;93:921-7; discussion 927-88. [PubMed]
- Dupuy DE, DiPetrillo T, Gandhi S, et al. Radiofrequency ablation followed by conventional radiotherapy for medically inoperable stage I non-small cell lung cancer. Chest 2006;129:738-45. [PubMed]
- Renaud S, Falcoz PE, Olland A, et al. Is radiofrequency ablation or stereotactic ablative radiotherapy the best treatment for radically treatable primary lung cancer unfit for surgery? Interact Cardiovasc Thorac Surg 2013;16:68-73. [PubMed]
- Crabtree T, Puri V, Timmerman R, et al. Treatment of stage I lung cancer in high-risk and inoperable patients: comparison of prospective clinical trials using stereotactic body radiotherapy (RTOG 0236), sublobar resection (ACOSOG Z4032), and radiofrequency ablation (ACOSOG Z4033). J Thorac Cardiovasc Surg 2013;145:692-9. [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [PubMed]
- Harada H, Okada M, Sakamoto T, et al. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005;80:2041-5. [PubMed]
- Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol 2014;32:2449-55. [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg 2013;146:358-64. [PubMed]
- Fernando HC, Timmerman R. American College of Surgeons Oncology Group Z4099/Radiation Therapy Oncology Group 1021: a randomized study of sublobar resection compared with stereotactic body radiotherapy for high-risk stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2012;144:S35-8. [PubMed]
- Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol 2010;28:928-35. [PubMed]
- Puri V, Crabtree TD, Kymes S, et al. A comparison of surgical intervention and stereotactic body radiation therapy for stage I lung cancer in high-risk patients: a decision analysis. J Thorac Cardiovasc Surg 2012;143:428-36. [PubMed]
- Verstegen NE, Oosterhuis JW, Palma DA, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol 2013;24:1543-8. [PubMed]
- Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2010;140:377-86. [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011;81:1352-8. [PubMed]
- Uematsu M, Shioda A, Suda A, et al. Computed tomography-guided frameless stereotactic radiotherapy for stage I non-small cell lung cancer: a 5-year experience. Int J Radiat Oncol Biol Phys 2001;51:666-70. [PubMed]
- Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:348-53. [PubMed]
- Louie AV, Rodrigues G, Hannouf M, et al. Stereotactic body radiotherapy versus surgery for medically operable Stage I non-small-cell lung cancer: a Markov model-based decision analysis. Int J Radiat Oncol Biol Phys 2011;81:964-73. [PubMed]
- Palma D, Lagerwaard F, Rodrigues G, et al. Curative treatment of Stage I non-small-cell lung cancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review. Int J Radiat Oncol Biol Phys 2012;82:1149-56. [PubMed]
- Guckenberger M, Baier K, Polat B, et al. Dose-response relationship for radiation-induced pneumonitis after pulmonary stereotactic body radiotherapy. Radiother Oncol 2010;97:65-70. [PubMed]
- Yorke ED, Jackson A, Rosenzweig KE, et al. Correlation of dosimetric factors and radiation pneumonitis for non-small-cell lung cancer patients in a recently completed dose escalation study. Int J Radiat Oncol Biol Phys 2005;63:672-82. [PubMed]
- Seppenwoolde Y, Lebesque JV, de Jaeger K, et al. Comparing different NTCP models that predict the incidence of radiation pneumonitis. Normal tissue complication probability. Int J Radiat Oncol Biol Phys 2003;55:724-35. [PubMed]
- Guckenberger M, Heilman K, Wulf J, et al. Pulmonary injury and tumor response after stereotactic body radiotherapy (SBRT): results of a serial follow-up CT study. Radiother Oncol 2007;85:435-42. [PubMed]
- Matsuo Y, Shibuya K, Nakamura M, et al. Dose--volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys 2012;83:e545-9. [PubMed]
- Ong CL, Palma D, Verbakel WF, et al. Treatment of large stage I-II lung tumors using stereotactic body radiotherapy (SBRT): planning considerations and early toxicity. Radiother Oncol 2010;97:431-6. [PubMed]
- Borst GR, Ishikawa M, Nijkamp J, et al. Radiation pneumonitis in patients treated for malignant pulmonary lesions with hypofractionated radiation therapy. Radiother Oncol 2009;91:307-13. [PubMed]
- Barriger RB, Forquer JA, Brabham JG, et al. A dose-volume analysis of radiation pneumonitis in non-small cell lung cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2012;82:457-62. [PubMed]
- Yamashita H, Nakagawa K, Nakamura N, et al. Exceptionally high incidence of symptomatic grade 2-5 radiation pneumonitis after stereotactic radiation therapy for lung tumors. Radiat Oncol 2007;2:21. [PubMed]
- Hof H, Herfarth KK, Münter M, et al. Stereotactic single-dose radiotherapy of stage I non-small-cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 2003;56:335-41. [PubMed]
- Nyman J, Johansson KA, Hultén U. Stereotactic hypofractionated radiotherapy for stage I non-small cell lung cancer--mature results for medically inoperable patients. Lung Cancer 2006;51:97-103. [PubMed]
- Takayama K, Nagata Y, Negoro Y, et al. Treatment planning of stereotactic radiotherapy for solitary lung tumor. Int J Radiat Oncol Biol Phys 2005;61:1565-71. [PubMed]
- Wulf J, Haedinger U, Oppitz U, et al. Stereotactic radiotherapy for primary lung cancer and pulmonary metastases: a noninvasive treatment approach in medically inoperable patients. Int J Radiat Oncol Biol Phys 2004;60:186-96. [PubMed]
- Gomez DR, Hunt MA, Jackson A, et al. Low rate of thoracic toxicity in palliative paraspinal single-fraction stereotactic body radiation therapy. Radiother Oncol 2009;93:414-8. [PubMed]
- Prendergast BM, Dobelbower MC, Bonner JA, et al. Stereotactic body radiation therapy (SBRT) for lung malignancies: preliminary toxicity results using a flattening filter-free linear accelerator operating at 2400 monitor units per minute. Radiat Oncol 2013;8:273. [PubMed]
- Guckenberger M, Kestin LL, Hope AJ, et al. Is there a lower limit of pretreatment pulmonary function for safe and effective stereotactic body radiotherapy for early-stage non-small cell lung cancer? J Thorac Oncol 2012;7:542-51. [PubMed]
- Stephans KL, Djemil T, Reddy CA, et al. Comprehensive analysis of pulmonary function Test (PFT) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J Thorac Oncol 2009;4:838-44. [PubMed]
- Bishawi M, Kim B, Moore WH, et al. Pulmonary function testing after stereotactic body radiotherapy to the lung. Int J Radiat Oncol Biol Phys 2012;82:e107-10. [PubMed]
- Stephans KL, Djemil T, Tendulkar RD, et al. Prediction of chest wall toxicity from lung stereotactic body radiotherapy (SBRT). Int J Radiat Oncol Biol Phys 2012;82:974-80. [PubMed]
- Dunlap NE, Cai J, Biedermann GB, et al. Chest wall volume receiving >30 Gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010;76:796-801. [PubMed]
- Woody NM, Videtic GM, Stephans KL, et al. Predicting chest wall pain from lung stereotactic body radiotherapy for different fractionation schemes. Int J Radiat Oncol Biol Phys 2012;83:427-34. [PubMed]
- Andolino DL, Forquer JA, Henderson MA, et al. Chest wall toxicity after stereotactic body radiotherapy for malignant lesions of the lung and liver. Int J Radiat Oncol Biol Phys 2011;80:692-7. [PubMed]
- Mutter RW, Liu F, Abreu A, et al. Dose-volume parameters predict for the development of chest wall pain after stereotactic body radiation for lung cancer. Int J Radiat Oncol Biol Phys 2012;82:1783-90. [PubMed]
- Creach KM, El Naqa I, Bradley JD, et al. Dosimetric predictors of chest wall pain after lung stereotactic body radiotherapy. Radiother Oncol 2012;104:23-7. [PubMed]
- Pettersson N, Nyman J, Johansson KA. Radiation-induced rib fractures after hypofractionated stereotactic body radiation therapy of non-small cell lung cancer: a dose- and volume-response analysis. Radiother Oncol 2009;91:360-8. [PubMed]
- Forquer JA, Fakiris AJ, Timmerman RD, et al. Brachial plexopathy from stereotactic body radiotherapy in early-stage NSCLC: dose-limiting toxicity in apical tumor sites. Radiother Oncol 2009;93:408-13. [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [PubMed]
- Song SY, Choi W, Shin SS, et al. Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer 2009;66:89-93. [PubMed]
- Miller KL, Shafman TD, Anscher MS, et al. Bronchial stenosis: an underreported complication of high-dose external beam radiotherapy for lung cancer? Int J Radiat Oncol Biol Phys 2005;61:64-9. [PubMed]
- Milano MT, Chen Y, Katz AW, et al. Central thoracic lesions treated with hypofractionated stereotactic body radiotherapy. Radiother Oncol 2009;91:301-6. [PubMed]
- Oshiro Y, Aruga T, Tsuboi K, et al. Stereotactic body radiotherapy for lung tumors at the pulmonary hilum. Strahlenther Onkol 2010;186:274-9. [PubMed]
- Joyner M, Salter BJ, Papanikolaou N, et al. Stereotactic body radiation therapy for centrally located lung lesions. Acta Oncol 2006;45:802-7. [PubMed]
- van der Voort van Zyp NC, Prévost JB, van der Holt B, et al. Quality of life after stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2010;77:31-7. [PubMed]
- Widder J, Postmus D, Ubbels JF, et al. Survival and quality of life after stereotactic or 3D-conformal radiotherapy for inoperable early-stage lung cancer. Int J Radiat Oncol Biol Phys 2011;81:e291-7. [PubMed]
- Lagerwaard FJ, Aaronson NK, Gundy CM, et al. Patient-reported quality of life after stereotactic ablative radiotherapy for early-stage lung cancer. J Thorac Oncol 2012;7:1148-54. [PubMed]
- Handy JR Jr, Asaph JW, Skokan L, et al. What happens to patients undergoing lung cancer surgery? Outcomes and quality of life before and after surgery. Chest 2002;122:21-30. [PubMed]
- Bolliger CT, Wyser C, Roser H, et al. Lung scanning and exercise testing for the prediction of postoperative performance in lung resection candidates at increased risk for complications. Chest 1995;108:341-8. [PubMed]
- Markos J, Mullan BP, Hillman DR, et al. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis 1989;139:902-10. [PubMed]
- Robnett TJ, Machtay M, Vines EF, et al. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys 2000;48:89-94. [PubMed]
- Swensen SJ, Silverstein MD, Ilstrup DM, et al. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med 1997;157:849-55. [PubMed]
- Herder GJ, Kramer H, Hoekstra OS, et al. Traditional versus up-front [18F] fluorodeoxyglucose-positron emission tomography staging of non-small-cell lung cancer: a Dutch cooperative randomized study. J Clin Oncol 2006;24:1800-6. [PubMed]
- Midthun DE, Swensen SJ, Jett JR. Clinical strategies for solitary pulmonary nodule. Annu Rev Med 1992;43:195-208. [PubMed]
- Henschke CI, Yankelevitz DF, Naidich DP, et al. CT screening for lung cancer: suspiciousness of nodules according to size on baseline scans. Radiology 2004;231:164-8. [PubMed]
- Swensen SJ, Jett JR, Sloan JA, et al. Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med 2002;165:508-13. [PubMed]
- Quint LE, Park CH, Iannettoni MD. Solitary pulmonary nodules in patients with extrapulmonary neoplasms. Radiology 2000;217:257-61. [PubMed]
- Patel VK, Naik SK, Naidich DP, et al. A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: part 1: radiologic characteristics and imaging modalities. Chest 2013;143:825-39. [PubMed]
- Patel VK, Naik SK, Naidich DP, et al. A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: part 2: pretest probability and algorithm. Chest 2013;143:840-6. [PubMed]
- Garland LH, Coulson W, Wollin E. The rate of growth and apparent duration of untreated primary bronchial carcinoma. Cancer 1963;16:694-707. [PubMed]
- Friberg S, Mattson S. On the growth rates of human malignant tumors: implications for medical decision making. J Surg Oncol 1997;65:284-97. [PubMed]
- Duhaylongsod FG, Lowe VJ, Patz EF Jr, et al. Lung tumor growth correlates with glucose metabolism measured by fluoride-18 fluorodeoxyglucose positron emission tomography. Ann Thorac Surg 1995;60:1348-52. [PubMed]
- Murai T, Shibamoto Y, Baba F, et al. Progression of non-small-cell lung cancer during the interval before stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2012;82:463-7. [PubMed]
- Patz EF Jr, Rossi S, Harpole DH Jr, et al. Correlation of tumor size and survival in patients with stage IA non-small cell lung cancer. Chest 2000;117:1568-71. [PubMed]
- Pantel K, Izbicki J, Passlick B, et al. Frequency and prognostic significance of isolated tumour cells in bone marrow of patients with non-small-cell lung cancer without overt metastases. Lancet 1996;347:649-53. [PubMed]
- Peck K, Sher YP, Shih JY, et al. Detection and quantitation of circulating cancer cells in the peripheral blood of lung cancer patients. Cancer Res 1998;58:2761-5. [PubMed]
- Cote RJ, Beattie EJ, Chaiwun B, et al. Detection of occult bone marrow micrometastases in patients with operable lung carcinoma. Ann Surg 1995;222:415-23; discussion 423-5. [PubMed]
- Verstegen NE, Lagerwaard FJ, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy following a clinical diagnosis of stage I NSCLC: comparison with a contemporaneous cohort with pathologically proven disease. Radiother Oncol 2011;101:250-4. [PubMed]
- Sawada S, Yamashita M, Komori E, et al. Evaluation of resected tumors that were not diagnosed histologically but were suspected of lung cancer preoperatively: PD1-3-5. J Thorac Oncol 2007;2:S422.
- Lardinois D, Weder W, Hany TF, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med 2003;348:2500-7. [PubMed]
- Aquino SL, Asmuth JC, Alpert NM, et al. Improved radiologic staging of lung cancer with 2-[18F]-fluoro-2-deoxy-D-glucose-positron emission tomography and computed tomography registration. J Comput Assist Tomogr 2003;27:479-84. [PubMed]
- Cerfolio RJ, Ojha B, Bryant AS, et al. The accuracy of integrated PET-CT compared with dedicated PET alone for the staging of patients with nonsmall cell lung cancer. Ann Thorac Surg 2004;78:1017-23; discussion 1017-23. [PubMed]
- Stiles BM, Servais EL, Lee PC, et al. Point: Clinical stage IA non-small cell lung cancer determined by computed tomography and positron emission tomography is frequently not pathologic IA non-small cell lung cancer: the problem of understaging. J Thorac Cardiovasc Surg 2009;137:13-9. [PubMed]
- Cerfolio RJ. Counterpoint: Despite staging inaccuracies, patients with non-small cell lung cancer are best served by having integrated positron emission tomography/computed tomography before therapy. J Thorac Cardiovasc Surg 2009;137:20-2. [PubMed]
- Wolthaus JW, Sonke JJ, van Herk M, et al. Comparison of different strategies to use four-dimensional computed tomography in treatment planning for lung cancer patients. Int J Radiat Oncol Biol Phys 2008;70:1229-38. [PubMed]
- Latifi K, Oliver J, Baker R, et al. Study of 201 non-small cell lung cancer patients given stereotactic ablative radiation therapy shows local control dependence on dose calculation algorithm. Int J Radiat Oncol Biol Phys 2014;88:1108-13. [PubMed]
- Huang K, Dahele M, Senan S, et al. Radiographic changes after lung stereotactic ablative radiotherapy (SABR)--can we distinguish recurrence from fibrosis? A systematic review of the literature. Radiother Oncol 2012;102:335-42. [PubMed]


