|
Cite this article as: Muley T, Herth FJF, Schnabel P,
Dienemann H, Meister M. From tissue to molecular
phenotyping: Pre-analytical requirements Heidelberg
Experience. Transl Lung Cancer Res 2012;1(2):111-121. DOI:
10.3978/j.issn.2218-6751.2011.12.07
Review Article
From tissue to molecular phenotyping: Pre-analytical requirements
Heidelberg experience
Thomas R. Muley1, Felix JF. Herth2, Philipp A. Schnabel3, Hendrik Dienemann4, Michael Meister1
1Translational Research Unit; 2Department of Pneumology and Respiratory Medicine, Thoraxklinik-Heidelberg gGmbH, University of Heidelberg, Germany; 3Institute of Pathology, University of Heidelberg, Germany; 4Department of Surgery, Thoraxklinik-Heidelberg gGmbH, University of Heidelberg, Germany
Thomas Muley, PhD. Translational Research Unit, Thoraxklinik-Heidelberg gGmbH, University of Heidelberg, Amalienstr. 569126 Heidelberg, Germany. Tel: +49 6221 396 1110; Fax: +49 6221396 1652. Email: thomas.muley@thoraxklinik-heidelberg.de.
|
|
Abstract
Lung cancer is a leading cause of tumor–related death worldwide through years. Efforts to individualize lung cancer therapy to improve prognosis nowadays employ molecular analyses besides routine histopathological examination of tissue samples. In general, tissues are provided by bronchoscopy, CT-guided procedures or surgery. The sequence of tissue removal, storage, and processing has a considerable impact on the success and reliability of subsequent molecular biological analyses and will supposedly also influence therapeutic decisions. There is still an ongoing need for updated statements about the minimal requirements of tissue sampling for molecular diagnosis at international level and for certified/accredited quality control programs of the sampling procedures. Several of these issues may have to be adjusted to the individual local conditions. We will present several aspects of experiences gained in Thoraxklinik at the University Hospital of Heidelberg (TK-HD) with pre-analytical tissue requirements.
Key words
Lung cancer; pre-analytical tissue; molecular phenotyping
Transl Lung Cancer Res Dec 26, 2011. DOI: 10.3978/j.issn.2218-6751.2011.12.07
|
|
Introduction
The prognostic situation in lung cancer has changed only marginally in the last 20-30 years ( 1, 2). The poor 5-year survival rate of 15% is due to the fact that most of the patients are diagnosed in an advanced stage. Currently only about 30% of patients with manifested non-small cell lung cancer (NSCLC) can be treated curatively with surgery. Even in localized stage I which accounts for approximately 10% of the overall lung cancer population, the 5 year survival rate is suboptimal at 60-70%. The unsatisfactory overall survival rate leads to the quest of prognostic and predictive factors which might help to identify patients at risk, and can be further used as a surrogate for specific therapeutic options i.e. targeted individualized therapies. With the advent of numerous new diagnostic techniques which help to identify driver mutations (for example EGFR, EM4-ALK) ( 3- 5) and recent advances in understanding molecular biology of lung cancer more than 200 new agents are under investigation in preclinical and clinical studies ( 6). In addition, new algorithms for the sub classification of lung cancer have been developed in recent years ( 7). As a consequence, there is a considerable increase in workload for pulmonary pathologists. Twenty years ago one of the major tasks was to differentiate between NSCLC and SCLC. Today, even sub classification within the major NSCLC subtypes adenocarcinoma, squamous cell carcinoma, large cell carcinoma is recommended since the prognosis might be considerably different between the subtypes ( 8). New diagnostic "omics" methods analyzing genetic/epigenetic factors, gene expression, and protein analysis by high throughput technologies may help to establish and to monitor individualized approaches of therapy ( 9- 12). The majority of these techniques require high quality tissue samples ( Table 1).
| |
|
|
| Table 1. Factors influencing the quality of molecular analyses. |
| Tissue sampling techniques/sources |
surgery, bronchoscopy, radiology |
| Tissue sampling conditions/ preservation |
amount, temperature, time, fresh frozen, fixation method |
| Tissue processing/ evaluation |
whole tissue, micro-; macro dissection, heterogeneity |
| Extraction of nucleic acids/treatment |
extraction methods, digestion of samples etc |
| Data analysis |
management of background, artifacts, and variations |
|
|
|
Sources
Tissue samples might be obtained from various sources i.e. bronchoscopy, radiology (for example by CT-guided FNA) and surgery. The major limiting factor is the amount of acquired
tissue ( Table 2).
| |
|
|
| Table 2. Number of biopsies necessary for accurate molecular diagnosis of the listed genes in respect to the various bronchoscopical
biopsy techniques. |
| |
21 g -NA |
19 g - NA |
NA |
14 g - NA |
TBB |
Cryo |
| Cell block |
| Number of biopsies |
4 |
4 |
4 |
2-3 |
4-5 |
4-5 |
| Kras mutation |
+ |
+ |
+ |
++ |
+ |
++ |
| EGFR mutation |
+ |
+ |
++ |
++ |
++ |
++ |
| TS (IHC/PCR) |
+ |
(+) |
+ |
++ |
++ |
++ |
| ERCC1 (IHC/PCR) |
- |
(+) |
+ |
++ |
+ |
++ |
| RRM1 PCR |
- |
(+) |
+ |
++ |
+ |
++ |
| EML4-ALK |
+ |
+ |
+ |
++ |
++ |
++ |
| Transbronchial biopsy (TBB), needle aspiration (NA), cryobiopsy (Cryo), immunhistochemistry (IHC), polymerase chain reaction (PCR) - not
suitable, (+) limited, + sufficient, ++ good. |
|
Transbronchial needle aspiration cytology
The diagnostic yield of conventional (non/ultrasound
guided) transbronchial needle aspiration (TBNA) is high for
endoscopically visible bronchial lesions (13). Conventional
TBNA has also been used for staging the mediastinum in lung
cancer since the early 80ties ( 14, 15). The diameter of the
needles is 19G or 21G. The 19G is preferred as tumor cells can
be sampled. The availability of the cytopathologist to assess the
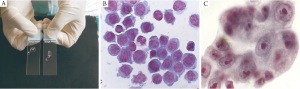
quality of the sample is helpful (Rapid onsite evaluation ROSE),
this increases the yield ( 16) ( Figure 1). Linear Endobronchial Ultrasound (EBUS)
In published meta-analysis EBUS-TBNA has been shown to
have a sensitivity above 9o% and high specificity of 100% ( 17).
Different publications have shown that, even in patients with
lymph nodes smaller than 1cm a significant percentage could
still be detected to have N2/N3 disease. Several studies have
evaluated the feasibility of analyzing gene alterations in lung
tumor tissue samples obtained by EBUS-TBNA. Determining
the EGFR mutation status in a series of EBUS-TBNA samples
was technically feasible in 26 out of the 36 (72.2%) patients.
Somatic mutations of the EGFR gene were detected in tissue
obtained through EBUS-TBNA in two (10%) out of 20 patients
with lung adenocarcinoma ( 18, 19). Endoscopic ultrasound (EUS)
EUS is especially useful for sampling posterior mediastinal
and paraoesophageal lymph nodes (stations 4L, 7, 8 and 9). In
addition, the left adrenal can be accessed ( 20). It has a so-called
‘seagull’ shape on ultrasound and is particularly well visualised in
cases of metastatic enlargement. EUS is more accurate and has
a higher predictive value than either PET or CT for posterior
mediastinal lymph nodes ( 21). The reproducibility of cytological
diagnoses on EBUS and EUS is good among experienced
cytopathologists. Combining EBUS and EUS
EBUS-TBNA and EUS-FNA have a complementary reach
for examining mediastinal nodes. EBUS has access to the
paratracheal, subcarinal and hilar regions and EUS to the
paraoesophageal lymph nodes. In combining these techniques,
all mediastinal lymph node stations (apart from stations 5 and 6)
as well as the left adrenal gland can be reached ( 22). For the EUS procedure the EBUS scope can be used ( 23). Bronchial and Transbronchial Biopsy
Biopsies of endobronchial Tumortumors have a yield of 75-
95% for diagnosis of malignancy ( 24). The larger the biopsy
sample, the more accurate the diagnosis. A larger number of
biopsy samples contributes to a more accurate diagnosis also
( 25, 26). In malignant bronchial biopsy samples, between
one third and one half of the biopsy fragments contain no
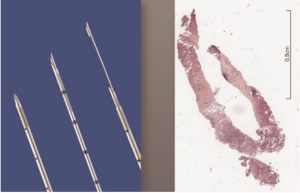
tumor ( 27). Cryobiopsies are a very effective technique to
produce large tumor biopsies with the potential to increase the
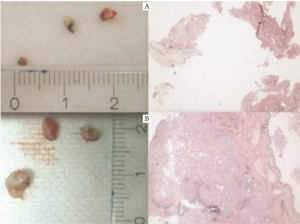
diagnostic yield at least in endobronchial tumors ( Figure 2-5)
( 28). In patients with solitary peripheral pulmonary nodules,
the endoscopic diagnostic procedure is usually performed as
transbronchial lung biopsy (TBBx) under fluoroscopic guidance.
This commonly performed procedure is associated with a low
yield in SPNs not visible by fluoroscopy ( 29). Normally 4 to 5
biopsies are taken. For lesions smaller than 3 cm a navigation
support based on a virtual bronchoscopy is recommended ( 30).
Using these systems herewith the yield for lesions around 2 cm
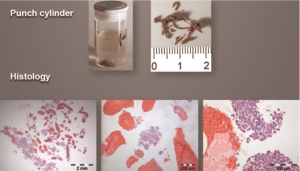
can be improved to 80 %. CT Guided Transthoracic needle biopsy (TTNBx)
For peripheral lesions transthoracic needle biopsy may be used.
The main indications for these techniques is to determine the
nature of a thoracic nodule or mass ( 31). TTNBx has an accuracy
varying between 80-95% ( 32, 33). The negative predictive value
of pulmonary biopsy is 84-96% and false negative results are
noted in 2-4% ( 34) CT-guided core needle biopsy and FNA
allows acquisition of material for predictive analysis using either
18-gauge or 20-gauge tru-cut biopsy needles via 17-gauge or
19-gauge coaxial needles ( 35, 36). Heidelberg recommendation
The best strategy to increase the yield of the bronchoscopic
samples is to combine several techniques. The optimal number
of needle passes should be three ore more. Rapid On Site
Examination (ROSE) is a quick cytological examination for the
presence of tumor or lymphoid cells by pathologist or trained
person ( 48). Initially, ROSE was set up for conventional (nonultrasound
guided) TBNA, for confirming the representativity of
the sample. However, with the aim of obtaining as much tumor
material as possible to allow more biomarker testing, the original
goal is redundant, and additional needle passes may be required
to obtain further tissue for molecular testing. Transbronchial
biopsy could be done in peripheral lesions to improve the
diagnostic yield of peripheral lesions. An upcoming alternative is
the use of a navigation system. Surgical options
A representative amount of tissue can be provided by
surgery, eg. open thoracotomy (wedge resection, lobectomy,
pneumonectomy), thoracoscopy or mediastinoscopy. However,
surgery is only possible in 30-40% of the patients. Several factors
influencing the quality of the tissue have to be considered: I. the
processing time which is in general longer than for biopsies and
II. the influence of general anesthesia and the "warm ischemia"
time on gene expression might be considerably high.
The interference of specific fixation methods with
downstream analytical methodology should be considered as
well (fresh frozen vs. FFPE or alternative fixation methods like
HOPE, PAXgene® tissue, RNA later and others)( 37- 41). In
addition, the determination of heterogeneity within the sample
is of great importance since the content of viable tumor cells,
stroma, necrosis and lung parenchyma may vary considerably
between patients and within patient sub samples ( Figure 6). Recently, Freidin and coworkers presented a study at the
WCLC 2011 in Amsterdam dealing with the effect of sampling
time, fixation method and storage temperature on quality
of extracted total RNA samples and the consecutive gene
expression profiles ( 37). Tumor tissue samples were taken
directly after chest opening I. immediately after lung resection
II. after transport to the pathology department III. and after
formalin fixation, paraffin embedding and long term storage IV.
The quality of isolated total RNA, reported as RNA Integrity
Number (RIN) ( 45) was fairly good for most fresh samples but
considerably worse for FFPE material. In addition, the number
of significantly expressed genes was comparable for most time
points and storage conditions with the exception of FFPE tissue.
In summary the best results were achieved when the tumor tissue
samples were taken shortly after chest opening.
Proceeding of the sampled material |
|
Proceeding of sampled material
Tissue banking
Tissue repositories are considered as an optimal source of fresh
frozen tissue samples at least for research purposes ( 41). The
TK-HD has built up a large lung tissue repository during the last
10 years ( Figure 7), which got accredited in 2010 as part of the
National Center for Tumor diseases (NCT)-tissue bank ( 42, 43).
Following these requirements SOP-guided quality-controlled
tissue procurement can be guaranteed. Besides fresh frozen
tissue, FFPE-tissue, multi tissue arrays and pathological platform
technologies may be provided via the NCT tissue bank. In addition, histological evaluations and quality controlled
nucleic acid extraction services are routinely provided for
scientists ( 46, 47). The biorepository can be linked to an in
house tumor documentation system, as a source of high quality
supervised clinical data. A strong interdisciplinary cooperation
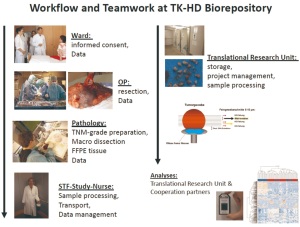
is essential for successful tissue procurement ( Figure 8). The
whole process starts at the ward by the patient’s informed
consent, which is a prerequisite. Basic clinical data enter the
clinical IT-system which employs a tumor documentation
system as an integral part. The tumor documentation follows the
recommendations of ADT ("working group of German tumor
centers") ( 44). During surgery all significant factors that may influence tissue
quality are documented i.e. time of chest opening, interruption
of blood supply, resection of the lung tissue, as well as
transportation time to the pathology lab. In our hands, freezing
of tissue samples can be accomplished within 15 to 30 minutes
after resection in the majority of cases.
At the pathology lab a TNM grade sample processing is
maintained and guaranteed even in small tumors (T1A). A high
quality of the tissue and high tumor content can be achieved
by macro-dissection of samples by an experienced pathologist.
However, this needs a good interaction with your pathologist.
Tissue samples are routinely divided in pieces of about 5×5×5
mm, distributed in labeled cryovials, weighted, and immediately
snap frozen in liquid nitrogen. Long term storage is performed at
-80°C in temperature monitored mechanical freezers.
Quality control of the banked samples
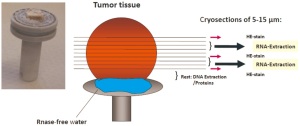
Before extraction of nucleic acids is performed, each individual
sample is evaluated by a standardized protocol for tumor cell
content as shown in Figure 9. One frozen piece of tumor tissue
is removed from a vial, attached to a cryostat chuck using sterile
RNAse-free water, and cut into 5-15 μm sections. .The first,
intermediate, and the last section of a series are Hematoxylin
and Eosin (H&E) stained and reviewed by a dedicated lung
pathologist to determine the proportion of viable tumor cell,
stromal cell, normal lung cell content, infiltrating lymphocytes
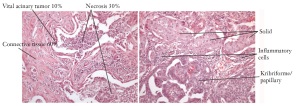
Figure 6. Heterogenity of tumor samples in respect to tumor cell content and sub types in resected adeno carcinoma of the
lung. Samples of two patients are shown as an example for heterogeneity within a tissue sample and between patients.
Kribriforme/
papillary
Vital acinary Tumor 10% Necrosis 30%
Connective tissue 60%
Solid
Inflammatory
cells and necrotic areas. The sections in between the stained sections
are transferred into pre-cooled micro vials and kept at -80°C
until nucleic acid extraction. For DNA/RNA extraction we use those tissue samples with
viable tumor content equal to or higher than 50%. Isolation
of nucleic acids is performed with commercially available
kits (AllPrep DNA/RNA kit, Qiagen, Hilden, Germany) and
adopted protocols, which allow us to extract DNA and total RNA
including miRNA in one session. The amount and quality of
nucleic acids are routinely checked with a NanoDrop ND-1000
Spectrophotometer (NanoDrop Technologies, Wilmington,
MA, USA) and an Agilent 2100 Bioanalyzer and Agilent RNA
6000 Nano Kit (Agilent Technologies, Boeblingen, Germany) (
Figure 10). On average the tumor content in a series of 447 selected
NSCLC tumor samples was 57.8 % ( Table 3). The isolation of nucleic acids from these samples resulted in an RNA quality
with a RNA-Integrity-Number (RIN) of approximately
9.0 ( 45). These samples are excellent templates for next
generation sequencing, mutational analyses, methylation
analyses, and microarray or qPCR based gene expression
analyses ( 46, 47). The average yield in DNA and RNA is
fairly high and was not a limiting factor for above molecular
biological analyses.
| |
|
|
| Table 3. Results of DNA and RNA extraction from lung cancer tissue samples (n=447) with an vital tumor cell content equal to or larger
than 50% using standardized nucleic acid extraction protocols. |
| |
Mean |
SD |
Median |
Min |
Max |
| DNA |
|
|
|
|
|
| OD 260/280 |
1.9 |
0.02 |
1.9 |
1.85 |
2.07 |
| Amount (μg) |
105.6 |
19.12 |
106.9 |
44.6 |
203.3 |
| Total RNA |
|
|
|
|
|
| RIN |
9.3 |
0.67 |
9.5 |
4.7 |
10 |
| Amount (μg) |
52.9 |
25.2 |
49.5 |
2.4 |
138.1 |
| Tumor cell |
|
|
|
|
|
| content (%) |
57.8 |
7.8 |
55 |
50 |
87.5 |
| RIN = RNA integrity number; OD = optical density. |
|
|
|
Conclusion
Various tissue sampling techniques are currently available and
most of them will result in a high diagnostic yield in lung cancer.
Nevertheless, the large variability in the number of cells and the
heterogeneity within a tumor sample itself represents a challenge
for molecular analyses. Therefore, not every tissue sampling
technique may be ideally suited for all kinds of marker analyses.
There is clearly an ongoing need for updated statements about
the minimal pre-analytical requirements of tissue sampling for molecular diagnosis on an international level. Additionally,
there is also an existing need for local adaptations of the
program and certificated or accredited quality control programs.
It is anticipated that the combination of classical clinical,
pathological, and molecular biology techniques will influence
the diagnosis and improve the treatment options of patients
suffering from lung cancer.
|
|
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin
2009;59:225-49.[LinkOut]
- Janssen-Heijnen ML, Coebergh JW. The changing epidemiology of lung
cancer in Europe. Lung Cancer 2003;41:245-58.[LinkOut]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal
growth factor receptor underlying responsiveness of non-small-cell lung
cancer to gefitinib. N Engl J Med 2004;350:2129-39.[LinkOut]
- Gerber DE, Minna JD. ALK inhibition for non-small cell lung cancer: from
discovery to therapy in record time. Cancer Cell 2010;18:548-51.[LinkOut]
- Petersen I. The morphological and molecular diagnosis of lung cancer.
Dtsch Arztebl Int 2011;108:525-31.[LinkOut]
- Somaiah N, Simon GR. Molecular targeted agents and biologic therapies
for lung cancer. J Thorac Oncol 2011;6:S1758-85.[LinkOut]
- Travis WD, Brambilla E, Noguchi M, et al. International association
for the study of lung cancer/american thoracic society/european
respiratory society international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol 2011;6:244-85.[LinkOut]
- Warth A, Muley T, Meister M, et al. Back to the Roots: The Novel
Histologic IASLC/ATS/ERS Classification System of Invasive Pulmonary
Adenocarcinoma is a Stage-Independent Predictor of Survival. J Clin Oncol
2011 (accepted). [Epub ahead of print][LinkOut]
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med
2008;359:1367-80.[LinkOut]
- Herbst RS, Lippman SM. Molecular signatures of lung cancer--toward
personalized therapy. N Engl J Med 2007;356:76-8.[LinkOut]
- Massion PP, Carbone DP. The molecular basis of lung cancer: molecular
abnormalities and therapeutic implications. Respir Res 2003;4:12.[LinkOut]
- Ross JS, Cronin M. Whole cancer genome sequencing by next-generation
methods. Am J Clin Pathol 2011;136:527-39.[LinkOut]
- Dasgupta A, Jain P, Minai OA, et al. Utility of transbronchial needle
aspiration in the diagnosis of endobronchial lesions. Chest 1999;115:1237-
41.[LinkOut]
- Punamiya V, Mehta A, Chhajed PN. Bronchoscopic needle aspiration in
the diagnosis of mediastinal lymphadenopathy and staging of lung cancer. J
Cancer Res Ther 2010;6:134-41.[LinkOut]
- Harrow EM, Abi-Saleh W, Blum J, et al. The utility of transbronchial needle
aspiration in the staging of bronchogenic carcinoma. Am J Respir Crit Care
Med 2000;161:601-7.[LinkOut]
- Hsu LH, Liu CC, Ko JS. Education and experience improve the
performance of transbronchial needle aspiration: a learning curve at a
cancer center. Chest 2004;125:532-40.[LinkOut]
- Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided
transbronchial needle aspiration for staging of lung cancer: a systematic
review and meta-analysis. Eur J Cancer 2009;45:1389-96.[LinkOut]
- Sakairi Y, Nakajima T, Yasufuku K, et al. EML4-ALK fusion gene
assessment using metastatic lymph node samples obtained by
endobronchial ultrasound-guided transbronchial needle aspiration. Clin
Cancer Res 2010;16:4938-45.[LinkOut]
- Nakajima T, Yasufuku K. How I do it--optimal methodology for
multidirectional analysis of endobronchial ultrasound-guided
transbronchial needle aspiration samples. J Thorac Oncol 2011;6:203-6.[LinkOut]
- Usuda J, Ichinose S, Ishizumi T, et al. Outcome of photodynamic therapy
using NPe6 for bronchogenic carcinomas in central airways >1.0 cm in
diameter. Clin Cancer Res 2010;16:2198-204.[LinkOut]
- Micames CG, McCrory DC, Pavey DA, et al. Endoscopic ultrasoundguided
fine-needle aspiration for non-small cell lung cancer staging: A
systematic review and metaanalysis. Chest 2007;131:539-48.[LinkOut]
- Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs
endosonography for mediastinal nodal staging of lung cancer: a randomized
trial. JAMA 2010;304:2245-52.[LinkOut]
- Herth FJ, Krasnik M, Kahn N, et al. Combined endoscopic-endobronchial
ultrasound-guided fine-needle aspiration of mediastinal lymph nodes
through a single bronchoscope in 150 patients with suspected lung cancer.
Chest 2010;138:790-4.[LinkOut]
- Ikeda N, Hayashi A, Iwasaki K, et al. Comprehensive diagnostic
bronchoscopy of central type early stage lung cancer. Lung Cancer
2007;56:295-302.[LinkOut]
- Gellert AR, Rudd RM, Sinha G, et al. Fibreoptic bronchoscopy: effect of
multiple bronchial biopsies on diagnostic yield in bronchial carcinoma.
Thorax 1982;37:684-7.[LinkOut]
- Popovich J Jr, Kvale PA, Eichenhorn MS, et al. Diagnostic accuracy of
multiple biopsies from flexible fiberoptic bronchoscopy. A comparison of
central versus peripheral carcinoma. Am Rev Respir Dis 1982;125:521-3.[LinkOut]
- Coghlin CL, Smith LJ, Bakar S, et al. Quantitative analysis of tumor in
bronchial biopsy specimens. J Thorac Oncol 2010;5:448-52.[LinkOut]
- Hetzel J, Eberhardt R, Herth FJ, et al. Cryobiopsy increases the diagnostic
yield of endobronchial biopsy: a multicentre trial. Eur Respir J 2011. [Epub
ahead of print][LinkOut]
- Baaklini WA, Reinoso MA, Gorin AB, et al. Diagnostic yield of fiberoptic
bronchoscopy in evaluating solitary pulmonary nodules. Chest
2000;117:1049-54.[LinkOut]
- Eberhardt R, Anantham D, Herth F, et al. Electromagnetic navigation
diagnostic bronchoscopy in peripheral lung lesions. Chest 2007;131:1800-5.[LinkOut]
- Ghaye B, Dondelinger RF. Imaging guided thoracic interventions. Eur
Respir J 2001;17:507-28.[LinkOut]
- Laurent F, Latrabe V, Vergier B, et al. Percutaneous CT-guided biopsy of
the lung: comparison between aspiration and automated cutting needles
using a coaxial technique. Cardiovasc Intervent Radiol 2000;23:266-72.[LinkOut]
- Levine MS, Weiss JM, Harrell JH, et al. Transthoracic needle aspiration
biopsy following negative fiberoptic bronchoscopy in solitary pulmonary
nodules. Chest 1988;93:1152-5.[LinkOut]
- Fassina A, Corradin M, Zardo D, et al. Role and accuracy of rapid on-site
evaluation of CT-guided fine needle aspiration cytology of lung nodules.
Cytopathology 2011;22:306-12.[LinkOut]
- Solomon SB, Zakowski MF, Pao W, et al. Core needle lung biopsy
specimens: adequacy for EGFR and KRAS mutational analysis. AJR Am J
Roentgenol 2010;194:266-9.[LinkOut]
- Cheung YC, Chang JW, Hsieh JJ, et al. Adequacy and complications of
computed tomography-guided core needle biopsy on non-small cell lung
cancers for epidermal growth factor receptor mutations demonstration:
18-gauge or 20-gauge biopsy needle. Lung Cancer 2010;67:166-9.[LinkOut]
- Freidin MB, Bhudia N, Lim E, et al. Sample handling and processing are
critical factors influencing the resultsof whole genome gene expression
profiling [abstract]. Lung Cancer 2011;6:S373-4.
- Olert J, Wiedorn KH, Goldmann T, et al. HOPE fixation: a novel fixing
method and paraffin-embedding technique for human soft tissues. Pathol
Res Pract 2001;197:823-6.[LinkOut]
- Goldmann T, Drömann D, Marzouki M, et al. Tissue microarrays from
HOPE-fixed specimens allow for enhanced high throughput molecular
analyses in paraffin-embedded material. Pathol Res Pract 2005;201:599-
602.[LinkOut]
- Gonzalez P, Zigler JS Jr, Epstein DL, et al. Identification and isolation of
differentially expressed genes from very small tissue samples. Biotechniques
1999;26:884-6, 888-92.[LinkOut]
- Lawson MH, Rassl DM, Cummings NM, et al. Tissue banking of diagnostic
lung cancer biopsies for extraction of high quality RNA. J Thorac Oncol
2010;5:956-63.[LinkOut]
- Herpel E, Koleganova N, Schirmacher P. [Tissue bank of the National
Centre for Tumour Disease. An innovative platform for translational
tumour]. Pathologe 2008;29:204-9.[LinkOut]
- Herpel E, Röcken C, Manke H, et al. Quality management and
accreditation of research tissue banks: experience of the National Center
for Tumor Diseases (NCT) Heidelberg. Virchows Arch 2010;457:741-7.[LinkOut]
- Recommendations for tumor documentation of the ADT ("working group
of German tumor centers"). Available online: http://www.tumorzentren.
de/onkol-basisdatensatz.html.
- Schroeder A, Mueller O, Stocker S, et al. The RIN: an RNA integrity
number for assigning integrity values to RNA measurements. BMC Mol
Biol 2006;7:3.[LinkOut]
- Kuner R, Muley T, Meister M, et al. Global gene expression analysis reveals
specific patterns of cell junctions in non-small cell lung cancer subtypes.
Lung Cancer 2009;63:32-8.[LinkOut]
- Singer S, Malz M, Herpel E, et al. Coordinated expression of stathmin
family members by far upstream sequence element-binding protein-1
increases motility in non-small cell lung cancer. Cancer Res 2009;69:2234-
43.[LinkOut]
- Lamprecht B, Porsch P, Pirich C, et al. Electromagnetic navigation
bronchoscopy in combination with PET-CT and rapid on-site
cytopathologic examination for diagnosis of peripheral lung lesions. Lung
2009;187:55-9.[LinkOut]
|