Chemotherapy of lung cancer: A global perspective of the role of ifosfamide
Introduction
Ifosfamide (IFO) has been evaluated intensively in bronchogenic carcinoma. Available data indicate that ifosfamide as a single agent can approximate a 50% objective response rate in small cell lung cancer (SCLC) and a 25% response rate in non-small cell lung cancer (NSCLC). With mesna uroprotection, the primary dose-limiting toxicity is myelosuppression. In combination with other active agents (Tables 1,2), ifosfamide has contributed to high response rates in both SCLC and NSCLC (1).
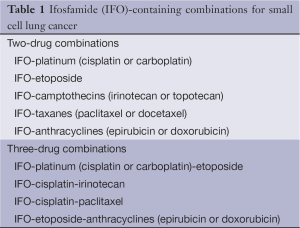
Full table
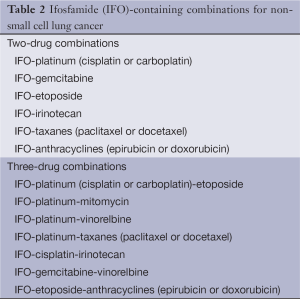
Full table
Preclinical evidence
The effects of IFO on human tumor xenografts were assessed in nude mice. Fifteen out of 43 tumors (36%) showed regression, among them 2/7 NSCLC and 3/4 SCLC (2).
SCLC xenografts were used to test the sensitivity to etoposide, cisplatin and ifosfamide as single agents and two- or three-drug combinations. IFO inhibited tumor growth in a dose-dependent manner, whereas cisplatin or etoposide alone had little or no effect. In two-drug combinations, each component potentiated the effects of the other, with etoposide/IFO being more effective than etoposide/cisplatin and better tolerated than cisplatin/IFO. The addition of a third agent gave a modest gain in therapeutic benefit at best (3). A panel of xenografts-three sensitive to a combination of etoposide, cisplatin, and IFO and three resistant-were exposed to topotecan. Growth inhibition was greater than 84% for five xenografts and IFO improved the efficacy of topotecan in 3/5 xenografts (4). Because a large proportion of SCLC tumors express c-kit, the therapeutic efficacy of imatinib, alone or combined with chemotherapy, was investigated in human SCLC xenografts. The efficacy of imatinib alone was variable but a significant increase of growth inhibition induced by etoposide-IFO or topotecan was observed (5).
IFO was one of the most effective cytostatics studied in NSCLC rat tumors (6). The effects of combined exposure of ionizing radiation and the in vitro active IFO metabolite 4-hydroperoxyifosfamide (4HOOIF) on cell survival were investigated in Caski squamous carcinoma and other cell lines. The combined exposure resulted in cell killing that was greater than for independent action. While radiosensitization was of minor magnitude for log-phase cells or cells in G1, substantial radiosensitization was detected for the most radioresistant S-phase cells (7).
Small cell lung cancer: the Western evidence
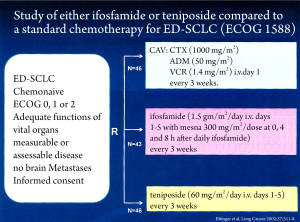
A randomized study of chemotherapy-naive patients with extensive disease SCLC compared IFO or teniposide given as single agents with combination chemotherapy (CAV: cyclophosphamide/doxorubicin/vincristine) (Figure 1). Among the 43 patients on ifosfamide, there were three complete and 18 partial responses (49%), while among the 46 patients on teniposide, there were two complete and 18 partial responses (43%). Two complete and 24 partial responses (56%) were seen among the 46 patients on CAV. The estimated median survival time was 43 weeks for IFO, 38 weeks for teniposide and 42 weeks for CAV. The treatments were significantly different with respect to the overall degree of toxicity with CAV being more toxic (8).
Platinum-etoposide (or irinotecan)-based regimens are currently the standard initial chemotherapy for most patients with SCLC (9). To determine whether the addition of IFO to cisplatin/etoposide (VP) improves results, patients with extensive SCLC were randomized between VP or cisplatin, IFO, and etoposide (VIP) in a study by the Hoosier Oncology Group. VIP was associated with significantly improved time to progression (P=0.039) and overall survival (P=0.045). The median survival times on VP and VIP were 7.3 months and 9.0 months, respectively. Myelosuppression was greater with VIP (10). Later on, British Medical Research Council multicenter randomized LU21 trial confirmed that survival could be improved by a similar regimen of IFO, carboplatin, etoposide, and mid-cycle vincristine (V-ICE) compared with standard chemotherapy in patients with good performance status. Four hundred and two patients were randomly assigned to receive six cycles of either V-ICE at 4-week intervals without dose reduction or standard chemotherapy (VP; or cyclophosphamide/doxorubicin/etoposide). Overall survival was longer in the V-ICE group (hazard ratio, 0.74; P=0.0049), median survival was 15.6 months in the V-ICE group and 11.6 months in the control group, and 2-year survival rates were 20% and 11%, respectively. The findings on quality of life were broadly similar in both groups, with some benefit in favor of V-ICE (11). Dose intensification of V-ICE from a 4-week to a 3-week schedule appeared to increase survival significantly compared with the standard arm (P=0.0014) in another randomized trial and was not associated with increased toxicity; median survival rates were 443 versus 351 days and 2-year survival rates were 33% versus 18%, respectively (12). A subsequent phase 3 study then assessed benefits of further dose intensification of ICE chemotherapy (ICT). Patients received up to six cycles of ICT with filgrastim-supported sequential reinfusion of peripheral blood progenitor cells every 14 days (n=42), or standard ICE (SCT) every 28 days (n=41). Median survival was significantly improved with ICT (30.3 months) versus SCT (18.5 months; P=0.001); 2-year survival was 55% for ICT and 39% for SCT (P=0.151). Time to progression (TTP) was also significantly improved (15 months for ICT versus 11.1 months for SCT, P=0.0001). ICT was associated with significantly more severe hematological toxicity and a significantly increased need for platelet and red blood cell transfusions (13). Further raising the dose intensity of ICE chemotherapy (by threefold) did not improve the long-term outcome of SCLC (limited or extensive with no more than two metastatic sites). In the randomized trial of the Solid Tumors Working Party of the European Group for Blood and Marrow Transplantation, the 3-year survival rates were 18% and 19% in the high-ICE and standard-ICE arms, respectively. No differences were observed in overall response (78% and 68%) or complete response (39% and 34%). High-ICE treatment was predictably associated with severe myelosuppression, and five patients (8%) died from toxicity (14).
Pursuing the goal of optimizing SCLC treatment, the European Lung Cancer Working Party (ELCWP) performed a randomized trial to determine if maintenance chemotherapy with etoposide/vindesine could improve progression-free survival of patients who responded to six courses of induction chemotherapy with ifosfamide, etoposide, and an anthracycline (doxorubicin or epirubicin). Among 235 eligible patients initially registered, 91 were randomized to receive maintenance therapy. Progression-free survival was significantly improved (P=0.003) by maintenance therapy, with median durations (maintenance vs. follow-up) of 25 versus 12 weeks after the second randomization. In a multivariate analysis, limited disease and maintenance were found to be independent positive predictors of survival (15). The Hoosier Oncology Group found in another phase III study that the addition of 3 months of oral etoposide in non-progressing patients with extensive SCLC treated with four cycles of VIP also improved progression-free survival (PFS) and overall survival. From 233 patients who were entered and treated with VIP, 144 non-progressing ones were subsequently randomized to oral etoposide (n=72) or observation (n=72). There was an improvement in median PFS (8.23 vs. 6.5 months) favor of the maintenance arm. There was also a trend towards an improvement in median (12.2 vs. 11.2 months), 1-year (51.4% vs. 40.3%), 2-year (16.7% vs. 6.9%) and 3-year survival (9.1% vs. 1.9%) in favoring the maintenance arm (16).
In SCLC, despite the high response rates induced by first-line chemotherapies, relapse occurs in the majority of the responding tumors. At the Montpellier Cancer Centre, Montpellier, France, a combination of epirubicin and ifosfamide (EI) has been developed as a non-cross-resistant salvage regimen for relapsed or refractory SCLC in the second and third line setting. Seventy patients were accrued; proportion of refractory, resistant and sensitive tumors was 20%, 21% and 59%, respectively. Forty-four patients were treated in second line setting whereas 26 have had already received two lines of prior therapy at time of accrual. Fifteen patients (21.4%) achieved an objective response (including one complete), and 10% had disease stabilization. Median overall survival was 3.9 months. NCI-CTC grade 3/4 toxicity was mainly haematological (17). In a phase II study from Greece, the PIC combination (paclitaxel/IFO/cisplatin) with granulocyte colony-stimulating factor support seemed highly active and tolerable in patients with relapsed SCLC when it was administered as second-line treatment after carboplatin/etoposide. Thirty-three patients were entered onto the study and eight complete remissions and 16 partial remissions could be induced (73% overall response rate). Median time to progression and overall survival were 21 weeks and 28 weeks, respectively. The 1-year survival rate was 12%. Grade 3/4 toxicities included neutropenia in 30 patients and febrile neutropenia in six; grade 3/4 thrombocytopenia was seen in nine patients (18).
Small cell lung cancer: The Asian evidence
In a Japanese study, a total of 36 SCLC patients were treated with IFO (without mesna uroprotection) as monotherapy. Fourteen (38.9%) achieved a clinical response, and 120 mg/kg of IFO was the minimum dose to obtain effectiveness. As side effects gastrointestinal disturbance (66.7%), depilation (66.7%), leukopenia (38.9%) and hematuria (36.1%) were reported. Mean survival time was prolonged by using IFO in comparison to groups treated with other anticancer drugs (19).
A total of 92 patients with SCLC were randomized to receive cisplatin/etoposide (PE) or cisplatin/etoposide/IFO (PEI) combination chemotherapy at the First Department of Medicine, Hokkaido University, Sapporo, Japan. No significant differences in the outcome were found for 89 evaluable patients; the overall response rates were 78% and 74% and the median survival times were 55 weeks and 56 weeks for PE and PEI therapy, respectively. Severe leukopenia occurred more often after PEI than after PE therapy (73% vs. 44%, P<0.05) (20). In a similar study performed at Xijing Hospital, Xi'an, Shaanxi, China, 120 chemotherapy-naive patients with localized SCLC were randomly divided to be treated with the VIP or the EP regimen, respectively. In 118 evaluable patients the overall response rates were similar (89.6% for VIP and 78.3% for EP), however, the complete response rate for the VIP regimen was significantly higher (43.1% vs. 25.0%, P<0.05). Toxicity of the two regimens was similar. The authors therefore conclude, that the VIP regimen may be used as the first-line chemotherapy for localized SCLC, and that its efficacy is superior to that of EP regimen (21). Supporting evidence for the benefit of treating SCLC with platinum/etoposide/IFO combinations also comes from two phase II studies from Thailand (22) and Korea (23). Twenty patients (8 LD, 12 ED) were treated with IFO/carboplatin/etoposide at Faculty of Medicine, Chiang Mai University, Thailand. Seventeen were evaluable and 14 (5 LD, 9 ED) achieved a partial response (82.5%). One-year survival was 23.5%. Because of severe myelosuppression, this regimen needed hematopoietic growth factor support, and after being prophylactically treated with GM-CSF, grade 3 and 4 neutropenia was reduced from 70.5% to 56.2%, 46.7%, 63.6%, 42.8% and 0% in cycle two to six, respectively (22). At College of Medicine, Hallym University, Seoul, Korea an etoposide/IFO/cisplatin (VIP) combination plus early concurrent thoracic irradiation was evaluated. Forty-four of the 49 patients who entered the study were evaluable and 28 (62%) showed a complete and 16 (38%) a partial response. The median survival time was 22.5 months. Twenty-four patients (54%) developed grade 3/4 neutropenia (23).
There is consensus that currently no standard second-line chemotherapy regimen exists for SCLC patients who fail to respond to initial treatment (refractory disease) or who relapse shortly after completion of first-line treatment (resistant disease with early relapse). Selected patients may benefit, however, from treatment with active agents not previously used, like amrubicin, topotecan, irinotecan, taxanes, gemcitabine ifosfamide, and oral etoposide (9). A phase II study with irinotecan/IFO as second-line chemotherapy for relapsed SCLC after prior chemotherapy including platinum/etoposide was conducted at Kurume University School of Medicine, Kurume, Japan. The combination demonstrated clinical efficacy with a favorable toxicity profile, particularly for performance status 0-1 and sensitive cases with only one metastatic site. Thirty-four patients were enrolled. The response rate was 52.9% with 2 complete responses and 16 partial responses. WHO grade 3/4 neutropenia was recorded in 52.9% of the patients, grade 3 diarrhea in 5.9% (24). The addition of cisplatin to IFO and irinotecan with rhG-CSF support led to highly active regimen for the treatment of refractory or relapsed SCLC. Eighteen patients entered a trial that was conducted at Minami-ichijo Hospital, Sapporo, Japan. There were 1 complete and 16 partial responses resulting in an overall response rate of 94.4%. The median survival time of all patients was 339 days, and the 1-year survival rate was 47.5%. Hematological toxicities were significant but diarrhea was mild and transient (25). The efficacy and safety of a paclitaxel plus ifosfamide (PI) salvage regimen in heavily pretreated SCLC patients was evaluated at the Samsung Medical Center, Seoul, Korea. Thirty-five (33 evaluable) patients who had received more than two prior chemotherapy regimens were treated. The overall response rate in the intent-to-treat population was 20.0% with 7 partial responses. Patients who responded to previous chemotherapy just before PI showed a significantly higher overall response rate than non-responders (57.1% versus 10.7%). After a median follow-up of 8.8 months, the median time to progression was 3.3 months and the median overall survival was 7.6 months. These findings suggest that PI salvage chemotherapy is a feasible and well tolerated regimen for extensively pretreated patients (26).
Non-small cell lung cancer: The Western evidence
It is currently agreed that four to six cycles of cisplatin-based chemotherapy for NSCLC patients with good performance status is associated with improved survival and symptom control. Chemotherapeutic regimens typically include cisplatin with at least one other active third generation drug (gemcitabine, paclitaxel, docetaxel, irinotecan and/or vinorelbine) or second generation drug (such as IFO, mitomycin C, vindesine, vinblastine). Chemotherapy based on drug triplets may be associated with higher tumor response rate and better overall survival at the expense of increased toxicity. Non-platinum-based regimens may be used in cases where platinum-based chemotherapy is contra-indicated. Single agent chemotherapy may still be considered in patients with poor performance status (27,28). IFO as a single agent has shown a response rate of 20-25%. These results are improved when it is used in combination with cisplatin and mitomycin C (MIC or MIP) or drugs like gemcitabine, taxanes and vinorelbine (29).
Preoperative (induction) chemotherapy was shown to improve clinical outcome of locally advanced NSCLC. In one of the first multicentric randomized studies, 60 patients were allocated to receive surgery alone or three cycles of MIC followed by surgery. All patients were scheduled to receive thoracic irradiation after surgery. For the 30 patients, each, who received chemotherapy or not, overall median survival was 22 months vs. 10 months (P=0.005). Updated survival data revealed a plateau in the chemotherapy group, suggesting that the natural history of still resectable NSCLC can be favorably altered by the multimodality therapeutic approach (30). Two large British randomized trials were performed to determine whether the addition of chemotherapy with the MIC regimen has an impact on duration and quality of life (QOL) in localized, unresectable (MIC1 trial) and extensive (MIC2 trial) NSCLC. Four hundred forty-six eligible ambulatory patients with localized disease were randomized in MIC1 to receive up to four cycles of chemotherapy followed by radical radiotherapy (CT+ RT) or radiotherapy (RT) alone. The median survival time was 11.7 months (CT + RT) versus 9.7 months (RT alone) (P=0.14). QOL showed improvement with chemotherapy and deterioration with standard treatment. In the combined analysis of 797 randomized patients, the positive effect of MIC on survival was significant overall (P=0.01) and after adjusting for prognostic factors (P=0.01) (31). The importance of dose-intensity has been analysed then in subsequent randomized trials. Patients with stage IIIA disease receive either high-dose cisplatin (HDCP, 100 mg/m2; n=46) or moderate-dose cisplatin (MDCP, 50 mg/m2; n=37) in combination with ifosfamide and mitomycin. Those patients with response or stable disease after three cycles underwent thoracotomy. Thoracotomy was performed in 71 patients (86%), 58 of whom had resectable disease. Complete resection rate was 61% in the HDCP group, and 51% in the MDCP group (P=0.5). A higher radiographic response rate was observed in patients who receive HDCP, but the study failed to show any significant improvement in either overall survival or pathologic complete response in that group (32). The role of chemotherapy dose intensity was also evaluated in another trial by testing two different induction chemotherapy regimens followed by thoracic irradiation. Chemotherapy consisted of three courses of MIP (mitomycin 6 mg/m2; IFO 3 g/m2; cisplatin 50 mg/m2) or SuperMIP (mitomycin 6 mg/m2; ifosfamide 4.5 g/m2; cisplatin 60 mg/m2, carboplatin 200 mg/m2). A total of 351 patients were eligible: 176 in the MIP arm and 175 in the SuperMIP arm. There was a significantly higher objective response rate with SuperMIP compared with MIP (46% vs. 35%, P=0.03) that did not translate, however, into a significant survival difference (P=0.16), with median survival times of 12.5 months and 11.2 months, for MIP and SuperMIP, respectively., Hematological toxicity and dosage reductions were higher with SuperMIP, which was nevertheless associated with significantly increased absolute dose intensity (33). The second generation drug mitomycin was replaced by the third generation drug vinorelbine in combination with cisplatin and IFO (NIP) in another phase III multinational trial (34), and at least two phase II trials studied the combination of paclitaxel/IFO/cisplatin (TIP) (35,36). After three cycles of NIP, patients were treated by surgery and within 45 days were randomized to two additional cycles of NIP versus observation. Overall, 155 patients received chemotherapy. After three cycles of induction in 143 assessable patients, 82 reported an objective response (57.3%). After a median of 32 days subsequent to NIP induction, 107 patients (69%) underwent operation with complete resection (R0) in 74% (79 of 107 patients). Seventy-nine patients were randomized to adjuvant NIP (47%) or control (53%). Overall median survival was 32.3 months versus 31.8 months in the observation and NIP arms, respectively (34). The Austrian Association for the Study of Lung Cancer (AASLC) included 47 patients in a multi-center phase II trial of TIP induction chemotherapy and prophylactic G-CSF. Forty-five patients were evaluable and an overall response rate of 43% (complete remission 4.5% and partial remission 38%) was achieved. Down-staging was achieved in 36% of the patients. No severe hematotoxicity was observed. Surgery was performed in 24 (51%) patients and resulted in complete tumor resection in 19. Median survival was 10.3 months for the total population and was longer for patients with down-staging as compared to those without and for patients with complete tumor resection as compared to the remaining ones (35). In another study, 35 patients received a course of 20 Gy of radiation in 2 weeks followed by two courses of TIP chemotherapy. Two to 3 weeks after chemotherapy, suitable patients underwent surgery. The regimen produced a high response rate with low treatment related morbidity/mortality. The overall response rate was 82.9% (20% CR). In 12 patients with stages IB, IIA and B, the median survival was 61 months, and 5-year survival was 55%, whereas in 23 patients with stages IIIA and B, the median survival was 26 months, and 5-year survival was 9.5%. There were 14 patients with grade 3/4 leukopenia. One patient died of grade 5 radiation pneumonitis. There was no postoperative death (36).
Two large, randomized, parallel trials were run in Great Britain to determine whether the addition of chemotherapy with the MIC regimen influences duration and quality of life in localized, unresectable (MIC1 trial, see above) and extensive (MIC2 trial) NSCLC. Three hundred fifty-five extensive-stage patients were randomized in MIC2 to chemotherapy plus palliative care (CT + PC) or palliative care (PC) alone. Chemotherapy significantly improved survival: median survival time was 6.7 months (CT + PC) compared with 4.8 months (PC alone) (P=0.03). Quality of life assessed from start of trial to week 6, showed improvement with chemotherapy and deterioration with standard treatment. In the combined analysis of 797 randomized patients, the positive effect of MIC on survival was significant overall (P=0.01) and after adjusting for prognostic factors (P=0.01) (31). The European Lung Cancer Working Party conducted a phase III randomized trial in a total of 305 patients with metastatic NSCLC, to determine if – in association with mitomycin and IFO – the combination of moderate dosages of cisplatin and carboplatin (CarboMIP regimen) could improved survival in comparison with cisplatin alone (MIP regimen). The trial failed to demonstrate significant differences in outcome between both arms; neither in the objective response rates (27% vs. 33%, P=0.34), nor in the duration of response or survival. Emesis, leukopenia and thrombocytopenia were significantly more severe in the CarboMIP arm (37). These results support the use of moderate-dose cisplatin in combination with IFO and mitomycin, and such regimens then have been compared in randomized phase trials to combinations containing a platinum and cytostatics of the third generation, like gemcitabine (38-40) or docetaxel (41). Gemcitabine and cisplatin (GC) was compared with MIC chemotherapy in a study of the Italian Lung Cancer Project recruiting 307 patients with stage IIIB or IV NSCLC. Although an increased response rate was reported for the GC arm (38% vs. 26%, P=0.029), that did not translate into improvements in quality of life, overall survival, time to progression, and time to treatment failure. Grade 3/4 thrombocytopenia was significantly worse in the GC arm (64% vs. 28%, P<0.001), whereas grade 3/4 alopecia was reported more commonly in the MIC arm (39% vs. 12%, P<0.001) (38). In a phase III randomized trial of the London Lung Cancer Group, gemcitabine was combined with carboplatin (GCa, n=212 patients) and compared with MIC (n=210 patients). Overall response rates were similar (42% for GCa vs. 41% for MIC; P=0.84), nonetheless there was a significant survival advantage for GCa compared with MIC (hazard ratio, 0.76; P=0.008). Median survival was 10 months with GCa and 7.6 months with MIC and 1-year survival was 40% with GCa and 30% with MIC. More thrombocytopenia occurred with GCa (P=0.03), but it caused less nausea, vomiting, constipation, and alopecia and was associated with fewer admissions for administration and better quality of life (39). Contrasting results were reported from a similar study with 372 patients randomized to receive gemcitabine plus carboplatin (GC) or MIC/MVP. There were no significant differences in median survival (MIC/MVP 248 days vs. GC 236 days) or time to progression (MIC/MVP 225 days vs. GC 218 days). The 1-and 2-year survival rates were 32.5% and 11.8% in the MIC/MVP arm and 33.2% and 6.9% in the GC arm, respectively. In the MIC/MVP arm, 33% of patients responded (4 complete and 57 partial responses) whereas in the GC arm, 30% of patients responded (3 complete and 54 partial responses). More alopecia was reported among patients in the MIC/MVP arm but GC appeared to produce more hematologic toxicity and necessitated more transfusions. There was no difference in performance status, disease-related symptoms, or quality of life between patients in the two treatment arms (40). No clinically significant differences in efficacy were also found in a randomized multicenter trial of the British Thoracic Oncology Group (BTOG1). Patients with stage III-IV NSCLC not suitable for curative surgery or radiotherapy received four cycles of either DCb (docetaxel and carboplatin), or MIC/MVP (mitomycin, cisplatin IFO or vinblastine, respectively). Overall response rate was 32% for both arms. One-year survival was 39% and 35% for DCb and MIC/MVP, respectively, and two-year survival was 13% with both arms. Grade 3/4 neutropenia (74% vs. 43%, P<0.005), infection (18% vs. 9%, P=0.01) and mucositis (5% vs. 1%, P=0.02) were more common with DCb than MIC/MVP. Quality of life was better maintained in the DCb arm (41).
A three-arm phase III randomized trial in stage IV NSCLC was organized by the European Lung Cancer Working Party to evaluate the effectiveness of non-platinum combinations. A total of 284 patients were randomized to be treated with the combination of gemcitabine/IFO (IG) or with a cisplatin/carboplatin association (CCG) or with a first-generation regimen of cisplatin/carboplatin/IFO (CCI). There were 94 eligible patients in the CCI arm, 92 in CCG and 94 in the IG arm. The objective response rates for CCI, CCG and IG were 23%, 29% and 25%, respectively (P=0.61). Median survival time was 24 weeks, 34 weeks and 30 weeks, respectively (P=0.20). One-year survival was 23%, 33% and 35%, and 2-year survival was 11%, 14% and 17%, respectively. In some subgroups (older patients, women), there was a significant survival advantage for CCG and IG compared with CCI (42). The activity and toxicity of a non platinum-based outpatient triplet of gemcitabine, IFO and vinorelbine (Navelbine) (GIN) in stage IIIB/IV NSCLC was investigated by the Italian Lung Cancer Task Force. Fifty patients entered the study. Twenty-five objective responses (1 complete and 24 partial) were obtained for a response rate of 52%. One-year survival was 46.5%. Neutropenia grade 3/4 occurred in 47% of the courses; thrombocytopenia grade 3/4 in 6.6%; anemia grade 3 in 3.5%. Twelve episodes of febrile neutropenia were reported and three patients required hospital admission. The authors discuss that the GIN regimen may represent a valuable alternative to standard platinum-based doublets and triplets in the treatment of advanced NSCLC (43).
Non-small cell lung cancer: the Asian evidence
A prospective randomized trial from India compared neoadjuvant chemotherapy followed by local-regional radiotherapy (study group) with radiotherapy alone (control group) in 506 patients with locally advanced NSCLC. The study group received three cycles of MIC. Radiotherapy was delivered up to a dose of 60 Gy. In the study group, 228 patients were assessed for tumor response after chemotherapy: 13 (5.7%) had a complete response, 103 (43.2%) a partial response and 48 (21%) showed no change. On completion of radiotherapy, 16.2% of the study group and 6% of the control group had a complete response. Actuarial 2-year survival was 20% in the study group and 7.4% in the control group (44).
The efficacy and toxicity of an IFO-cisplatin (IP) doublet in the treatment of advanced NSCLC was reported for 56 patients that were treated at Shanxi Provincial Cancer Hospital, China. The overall response rate was 50.0%, 52.8% for chemotherapy-naive patients and 45.0% for pretreated ones. The median relapse-free period was 5 months and the median duration of survival (MDS) was 9 months. The major toxicity was inhibition of bone marrow, especially of leukocytes and platelets (45). In another study from Hospital 309 of PLA, Beijing, China, the efficacy and safety of the IP regimen and of vinorelbine (Navelbine) combined with IFO and cisplatin (NIP regimen) was compared. One hundred and twenty patients with advanced NSCLC were randomly allocated to the IP regimen (n=60, 59 evaluable) or to the NIP regimen (n=60, 58 evaluable). For the IP doublet the response rates were 40.7% (24/59), 63.3% (19/30) and 17.2% (5/29) in whole group, untreated patients, and pretreated patients, respectively. The median duration of survival was 9 months and 1-year survival rate was 36.7%. For the NIP triplet the corresponding response rates were 58.6% (34/58), 65.6% (17/26) and 53.1% (17/32), respectively. The median duration of survival was 11.3 months and 1-year survival rate was 40.0%. The main dose limiting toxicity was myelosuppression. Leukopenia at grade III+IV was significantly higher in the NIP arm than in the IP arm (P<0.05). The authors conclude that IP regimen shows a similar response rate and less toxicity in chemotherapy-naive patients, compared with NIP regimen, and might be considered a relevant regimen for initial chemotherapy (46).
As already outlined for the treatment strategy in Western countries various triple combinations adding second (e.g., mitomycin) and third generation cytostatics (e.g., vinorelbine or irinotecan) to the platinum-IFO backbone have also been studied in Asian countries. A total of 206 patients with advanced unresectable NSCLC stage IIIB or IV were enrolled at Postgraduate Institute of Medical Education and Research, Chandigarh, India, to receive MIC combination chemotherapy. Nearly half of all followed-up patients showed a partial or complete radiological response. Overall performance status improved in 44 (30.8%) patients and worsened in 28 (19.6%). Overall median survival was 20 weeks, however, overall survival improved progressively with the number of chemotherapy cycles administered. Median survival in patients receiving at least three, four and five chemotherapy cycles was 23, 27 and 35 weeks, respectively. The side effects were minimal and acceptable, and the regimen was tolerated well by all the patients (47). Good tolerability and efficacy with objective responses in the range of 30% to 50% and median survival times around 7-11 months were also reported for a total of 94 patients treated with MIC/MIP regimens in smaller studies from China (48), Korea (49), and Singapore (50). The efficacy and toxicities of combinations using different doses of vinorelbine (25 mg/m2 or 20 mg/m2, days 1+5) and ifosfamide (3.0 g/m2 or 2.5 g/m2, day 5) with a constant dose of cisplatin (80 mg/m2 on day 5) was compared in a study from Yonsei University College of Medicine, Seoul, Korea. Twenty patients in arm A and 35 patients in arm B were evaluable. The response rate was 50% in arm A and 30% in arm B. The median survival times for arm A and B were 40 and 42 weeks, respectively. Leukopenia grade > III was observed in 28.9% in arm A and 17.2% in arm B. There was a significant correlation between the cumulative dose intensity and response rates and median survival (51). At Shanghai Chest Hospital, Shanghai, China gemcitabine and cisplatin (GP) was compared with vinorelbine, ifosfamide and cisplatin (NIP) combined chemotherapy. Eighty patients were included. The objective response rate was 40.0% in GP group, compared with 52.5% in NIP group (P>0.05). Median survival time of GP and NIP groups was 13.7 and 15.3 months, respectively, and 1-year survival rates were 54.3% and 59.5%, respectively (P>0.05). Leukopenia grade III/IV was significantly lower in GP arm (27.5% vs. 55.0%, P<0.05). In the opinion of the authors, GP may be a standard protocol for chemotherapy of advanced NSCLC whereas NIP should be given to young patients with good performance status (52). The combination of cisplatin, ifosfamide, and irinotecan with recombinant human granulocyte colony stimulating factor support was studied in three consecutive clinical trials at Minami-ichijo Hospital, Sapporo, Japan, and found highly effective with acceptable toxicities. Fifty patients were registered in a phase II study of which 49 were assessable for toxicity and response and 50 for survival. Thirty-three patients (67.3%) achieved an objective response. The median response duration was 192 days and the median time to progression was 170 days. The median survival time was 540 days with 1- and 2-year survival rates of 63.5% and 30.7%, respectively. Grade 3 or 4 neutropenia and thrombocytopenia developed in 63.3% and 38.8% of the patients, respectively (53). Both response rates and survival data that were analyzed retrospectively in patients with malignant pleural effusions and brain metastases also suggest a high degree of activity of the combination in these therapeutic situations (54,55).
Another phase II study that was conducted at Kurume University School of Medicine, Kurume, Japan, also demonstrated anti-tumor activity of a platinum-free two-drug combination of irinotecan and ifosfamide as first-line chemotherapy for stage IIIB or IV NSCLC, with response and survival rates similar to those of cisplatin-based chemotherapy but with a more favorable toxicity profile. Forty-four patients were enrolled and the response rate was 29.5%, with 13 partial responses. The median survival was 12.5 months, the median time to progression was 5.3 months, and the 1 and 2-year survival rates were 52.3% and 11.3%, respectively. WHO grade 3/4 neutropenia was recorded in 38.6% of the patients, grade 3 diarrhea in 6.8%, and grade 3/4 nausea/vomiting in 0% (56).
Conclusions: a look forward
Despite the progress achieved with chemotherapy, the long-term prognosis is still poor for the majority of patients with lung cancer.
For SCLC chemotherapy stagnates since years being based on platinum/etoposide doublets (cisplatin: VP/EP; or carboplatin: EC) as the standard. However, the addition of IFO to such doublets resulted in improved efficacy in a number of randomized studies (10,11,21) and moderate dose intensification of IFO-containing triplets, although associated with more severe hematological toxicity, increased survival further (12,13). Therefore, appropriately dosed platinum/etoposide/IFO-containing combinations seem to be one promising strategy to bring forward the first-line chemotherapy of SCLC-in particular for patients with localized disease and in good performance status. Currently no second-line chemotherapy regimen is accepted as standard after failure or relapse to initial treatment. Nonetheless, selected patients still may benefit from treatment with active agents not previously used (9) -and several IFO-containing regimens may meet these demands. Amongst others, combinations based on irinotecan/IFO have demonstrated considerable clinical efficacy with a acceptable toxicity profiles for relapsed or refractory SCLC (24,25) and a paclitaxel/IFO (PI) salvage regimen proved to be a feasible and well tolerated regimen for extensively pretreated patients (26). Finally, because a large proportion of SCLC tumors express c-kit, the therapeutic efficacy of the targeted agent imatinib was investigated in human SCLC xenografts. Its efficacy was variable but a significant increase of growth inhibition induced by etoposide/IFO or topotecan was observed (5), an observation worth substantiation in future clinical trials.
Platinum-based combinations are the currently agreed standard regimens for NSCLC resulting in improved survival and symptom control for patients with good performance status. They typically include at least one other active third generation drug (gemcitabine, paclitaxel, docetaxel, irinotecan and/or vinorelbine) or second generation drug (such as IFO, mitomycin C, vindesine, vinblastine). Chemotherapy based on drug triplets may be associated with higher tumor response rate and better overall survival at the expense of increased toxicity. Non-platinum-based regimens may be used with similar survival rates in cases where platinum-based chemotherapy is contra-indicated (28,57). The non-platinum-based outpatient triplet of gemcitabine, IFO and vinorelbine (Navelbine) (GIN) or the platinum-free two-drug combination of irinotecan and IFO represent valuable alternatives to standard platinum-based doublets and triplets with response and survival rates similar to those of cisplatin-based chemotherapy but with more favorable toxicity profiles (43,57). A comparison with standard regimens in randomized trials should clarify their position.
Finally, encouraging results have been achieved with IFO in a number of rare pulmonary tumors: Patients with pulmonary pleomorphic carcinoma (PPC) often have recurrent disease after surgery or metastatic disease and then have extremely poor responses to chemotherapy regimens commonly used for NSCLC. Contrary, two consecutive patients with platinum-refractory PPC that received doxorubicin/IFO/dacarbazine showed dramatic responses to this combination chemotherapy and the treatment effect was sustained for 7 and 9 months, respectively (58). Pleuropulmonary blastoma (PPB) is another rare and aggressive malignant tumor of the lung that is predominantly seen in children and is responsive to IFO-containing combinations like IFO/carboplatin/etoposide (59), IFO/vincristine (VCR)/ dactinomycin (60), IFO/VCR/ epirubicin (61) or IFO/doxorubicin (62).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Johnson DH. Overview of ifosfamide in small cell and non-small cell lung cancer. Semin Oncol 1990;17:24-30. [PubMed]
- Berger DP, Fiebig HH, Winterhalter BR, et al. Preclinical phase II study of ifosfamide in human tumour xenografts in vivo. Cancer Chemother Pharmacol 1990;26:S7-11. [PubMed]
- Nemati F, Livartowski A, De Cremoux P, et al. Distinctive potentiating effects of cisplatin and/or ifosfamide combined with etoposide in human small cell lung carcinoma xenografts. Clin Cancer Res 2000;6:2075-86. [PubMed]
- Némati F, Daniel C, Arvelo F, et al. Clinical relevance of human cancer xenografts as a tool for preclinical assessment: example of in-vivo evaluation of topotecan-based chemotherapy in a panel of human small-cell lung cancer xenografts. Anticancer Drugs 2010;21:25-32. [PubMed]
- Decaudin D, de Cremoux P, Sastre X, et al. In vivo efficacy of STI571 in xenografted human small cell lung cancer alone or combined with chemotherapy. Int J Cancer 2005;113:849-56. [PubMed]
- Kal HB, Meijnders PJ, Van Berkel AH, et al. Response to chemotherapy of non-small cell bronchial rat tumours growing subcutaneously or in the lung. In Vivo 1991;5:301-6. [PubMed]
- Latz D, Schulze T, Manegold C, et al. Combined effects of ionizing radiation and 4-hydroperoxyfosfamide in vitro. Radiother Oncol 1998;46:279-83. [PubMed]
- Ettinger DS, Finkelstein DM, Ritch PS, et al. Study of either ifosfamide or teniposide compared to a standard chemotherapy for extensive disease small cell lung cancer: an Eastern Cooperative Oncology Group randomized study (E1588). Lung Cancer 2002;37:311-8. [PubMed]
- Stahel R, Thatcher N, Früh M, et al. 1st ESMO Consensus Conference in lung cancer; Lugano 2010: small-cell lung cancer. Ann Oncol 2011;22:1973-80. [PubMed]
- Loehrer PJ Sr, Ansari R, Gonin R, et al. Cisplatin plus etoposide with and without ifosfamide in extensive small-cell lung cancer: a Hoosier Oncology Group study. J Clin Oncol 1995;13:2594-9. [PubMed]
- Thatcher N, Qian W, Clark PI, et al. Ifosfamide, carboplatin, and etoposide with midcycle vincristine versus standard chemotherapy in patients with small-cell lung cancer and good performance status: clinical and quality-of-life results of the British Medical Research Council multicenter randomized LU21 trial. J Clin Oncol 2005;23:8371-9. [PubMed]
- Steward WP, von Pawel J, Gatzemeier U, et al. Effects of granulocyte-macrophage colony-stimulating factor and dose intensification of V-ICE chemotherapy in small-cell lung cancer: a prospective randomized study of 300 patients. J Clin Oncol 1998;16:642-50. [PubMed]
- Buchholz E, Manegold C, Pilz L, et al. Standard versus dose-intensified chemotherapy with sequential reinfusion of hematopoietic progenitor cells in small cell lung cancer patients with favorable prognosis. J Thorac Oncol 2007;2:51-8. [PubMed]
- Leyvraz S, Pampallona S, Martinelli G, et al. A threefold dose intensity treatment with ifosfamide, carboplatin, and etoposide for patients with small cell lung cancer: a randomized trial. J Natl Cancer Inst 2008;100:533-41. [PubMed]
- Sculier JP, Paesmans M, Bureau G, et al. Randomized trial comparing induction chemotherapy versus induction chemotherapy followed by maintenance chemotherapy in small-cell lung cancer. European Lung Cancer Working Party. J Clin Oncol 1996;14:2337-44. [PubMed]
- Hanna NH, Sandier AB, Loehrer PJ Sr, et al. Maintenance daily oral etoposide versus no further therapy following induction chemotherapy with etoposide plus ifosfamide plus cisplatin in extensive small-cell lung cancer: a Hoosier Oncology Group randomized study. Ann Oncol 2002;13:95-102. [PubMed]
- Jacot W, Pujol JL, Chakra M, et al. Epirubicin and ifosfamide in relapsed or refractory small cell lung cancer patients. Lung Cancer 2012;75:213-6. [PubMed]
- Kosmas C, Tsavaris NB, Malamos NA, et al. Phase II study of paclitaxel, ifosfamide, and cisplatin as second-line treatment in relapsed small-cell lung cancer. J Clin Oncol 2001;19:119-26. [PubMed]
- Mukouda K, Morikawa E, Hasegawa K, et al. Gan To Kagaku Ryoho 1983;10:1293-8. [PubMed]
- Miyamoto H, Nakabayashi T, Isobe H, et al. A phase III comparison of etoposide/cisplatin with or without added ifosfamide in small-cell lung cancer. Oncology 1992;49:431-5. [PubMed]
- Wu C, Qi H, Dai Y, et al. Therapeutic efficacy of chemotherapy with VIP for small cell lung cancer. Zhongguo Fei Ai Za Zhi 2004;7:151-3. [PubMed]
- Thongprasert S. Phase II study of ifosfamide, carboplatin, etoposide and GM-CSF in small cell lung cancer. J Med Assoc Thai 2000;83:549-53. [PubMed]
- Woo IS, Park YS, Kwon SH, et al. A phase II study of VP-16-fosfamide-cisplatin combination chemotherapy plus early concurrent thoracic irradiation for previously untreated limited small cell lung cancer. Jpn J Clin Oncol 2000;30:542-6. [PubMed]
- Ichiki M, Gohara R, Rikimaru T, et al. Combination chemotherapy with irinotecan and ifosfamide as second-line treatment of refractory or sensitive relapsed small cell lung cancer: a phase II study. Chemotherapy 2003;49:200-5. [PubMed]
- Fujita A, Takabatake H, Tagaki S, et al. Combination of cisplatin, ifosfamide, and irinotecan with rhG-CSF support for the treatment of refractory or relapsed small-cell lung cancer. Oncology 2000;59:105-9. [PubMed]
- Park S, Ahn MJ, Ahn JS, et al. Combination chemotherapy with paclitaxel and ifosfamide as the third-line regimen in patients with heavily pretreated small cell lung cancer. Lung Cancer 2007;58:116-22. [PubMed]
- Azim HA Jr, Elattar I, Loberiza FR Jr, et al. Third generation triplet cytotoxic chemotherapy in advanced non-small cell lung cancer: a systematic overview. Lung Cancer 2009;64:194-8. [PubMed]
- Meert AP. Stage IV NSCLC. Place of chemotherapy. Rev Mal Respir 2008;25:3S107-12.
- Boni C, Zanelli F. Ifosfamide in non-small cell lung cancer. Oncology 2003;65:50-4. [PubMed]
- Rosell R, Gómez-Codina J, Camps C, et al. Preresectional chemotherapy in stage IIIA non-small-cell lung cancer: a 7-year assessment of a randomized controlled trial. Lung Cancer 1999;26:7-14. [PubMed]
- Cullen MH, Billingham LJ, Woodroffe CM, et al. Mitomycin, ifosfamide, and cisplatin in unresectable non-small-cell lung cancer: effects on survival and quality of life. J Clin Oncol 1999;17:3188-94. [PubMed]
- Felip E, Rosell R, Alberola V, et al. Preoperative high-dose cisplatin versus moderate-dose cisplatin combined with ifosfamide and mitomycin in stage IIIA (N2) non small-cell lung cancer: results of a randomized multicenter trial. Clin Lung Cancer 2000;1:287-93. [PubMed]
- Sculier JP, Lafitte JJ, Berghmans T, et al. A phase III randomised study comparing two different dose-intensity regimens as induction chemotherapy followed by thoracic irradiation in patients with advanced locoregional non-small-cell lung cancer. Ann Oncol 2004;15:399-409. [PubMed]
- Gottfried M, Ramlau R, Krzakowski M, et al. Cisplatin-based three drugs combination (NIP) as induction and adjuvant treatment in locally advanced non-small cell lung cancer: final results. J Thorac Oncol 2008;3:152-7. [PubMed]
- Pohl G, Krajnik G, Malayeri R, et al. Induction chemotherapy with the TIP regimen (paclitaxel/ifosfamide/cisplatin) in stage III non-small cell lung cancer. Lung Cancer 2006;54:63-7. [PubMed]
- Takita H, Shin KH, Soh AY, et al. Induction therapy of loco-regional non-small-cell lung cancer with reliable response and low toxicity (low dose radiotherapy sensitizes tumor to subsequent chemotherapy?). Lung Cancer 2009;63:387-92. [PubMed]
- Sculier JP, Lafitte JJ, Paesmans M, et al. Phase III randomized trial comparing moderate-dose cisplatin to combined cisplatin and carboplatin in addition to mitomycin and ifosfamide in patients with stage IV non-small-cell lung cancer. Br J Cancer 2000;83:1128-35. [PubMed]
- Crinò L, Scagliotti GV, Ricci S, et al. Gemcitabine and cisplatin versus mitomycin, ifosfamide, and cisplatin in advanced non-small-cell lung cancer: A randomized phase III study of the Italian Lung Cancer Project. J Clin Oncol 1999;17:3522-30. [PubMed]
- Rudd RM, Gower NH, Spiro SG, et al. Gemcitabine plus carboplatin versus mitomycin, ifosfamide, and cisplatin in patients with stage IIIB or IV non-small-cell lung cancer: a phase III randomized study of the London Lung Cancer Group. J Clin Oncol 2005;23:142-53. [PubMed]
- Danson S, Middleton MR, O'Byrne KJ, et al. Phase III trial of gemcitabine and carboplatin versus mitomycin, ifosfamide, and cisplatin or mitomycin, vinblastine, and cisplatin in patients with advanced nonsmall cell lung carcinoma. Cancer 2003;98:542-53. [PubMed]
- Booton R, Lorigan P, Anderson H, et al. A phase III trial of docetaxel/carboplatin versus mitomycin C/ifosfamide/cisplatin (MIC) or mitomycin C/vinblastine/cisplatin (MVP) in patients with advanced non-small-cell lung cancer: a randomised multicentre trial of the British Thoracic Oncology Group (BTOG1). Ann Oncol 2006;17:1111-9. [PubMed]
- Sculier JP, Lafitte JJ, Lecomte J, et al. A three-arm phase III randomised trial comparing combinations of platinum derivatives, ifosfamide and/or gemcitabine in stage IV non-small-cell lung cancer. Ann Oncol 2002;13:874-82. [PubMed]
- Baldini E, Ardizzoni A, Prochilo T, et al. Gemcitabine, Ifosfamide and Navelbine (GIN): activity and safety of a non-platinum-based triplet in advanced non-small-cell lung cancer (NSCLC). Br J Cancer 2001;85:1452-5. [PubMed]
- Sharma S, Sharma R, Bhowmik KT. Sequential chemoradiotherapy versus radiotherapy in the management of locally advanced non-small-cell lung cancer. Adv Ther 2003;20:14-9. [PubMed]
- Han F, Yang W, Zhu H, et al. Clinical study of ifosfamide and cisplatin in the treatment of advanced non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 2002;5:61-3. [PubMed]
- Liao G, Wang H, Qu Y, Liu P, et al. Comparison of navelbine plus ifosfamide and cisplatin versus ifosfamide plus cisplatin in the treatment of advanced non small cell lung cancer. Zhongguo Fei Ai Za Zhi 2003;6:138-40. [PubMed]
- Behera D, Aggarwal AN, Sharma SC, et al. Ifosfamide containing regimen for non-small cell lung cancer. Indian J Chest Dis Allied Sci 2004;46:9-15. [PubMed]
- Lin YC, Lin Y, Lin W, et al. Comparison of effects of three chemotherapy regimens for advanced non-small cell lung cancer. Ai Zheng 2002;21:1359-61. [PubMed]
- Han JY, Kim HK, Choi BG, et al. Quality of life (QOL) assessment of MIP (mitomycin, ifosfamide and cisplatin) chemotherapy in advanced non-small cell lung cancers (NSCLC). Jpn J Clin Oncol 1998;28:749-53. [PubMed]
- Soh LT, Tan EH, Ang PT. Mitomycin, ifosfamide and cisplatin in advanced non-small cell lung cancer. Singapore Med J 1998;39:357-8. [PubMed]
- Ahn JB, Ko WK, Lee JG, et al. Effect of vinorelbine, ifosfamide, and cisplatin combination chemotherapy in advanced non-small-cell lung cancer. Am J Clin Oncol 2000;23:622-8. [PubMed]
- Zhao Y, Chen Y, Ji H, et al. Comparative study on gemcitabine plus cisplatin and vinorelbine plus ifosfamide plus cisplatin combined chemotherapy in the treatment of advanced non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 2004;7:449-51. [PubMed]
- Fujita A, Ohkubo T, Hoshino H, et al. Phase II study of cisplatin, ifosfamide, and irinotecan with rhG-CSF support in patients with stage IIIb and IV non-small-cell lung cancer. Br J Cancer 2003;89:1008-12. [PubMed]
- Fujita A, Takabatake H, Tagaki S, et al. Combination chemotherapy in patients with malignant pleural effusions from non-small cell lung cancer: cisplatin, ifosfamide, and irinotecan with recombinant human granulocyte colony-stimulating factor support. Chest 2001;119:340-3. [PubMed]
- Fujita A, Fukuoka S, Takabatake H, et al. Combination chemotherapy of cisplatin, ifosfamide, and irinotecan with rhG-CSF support in patients with brain metastases from non-small cell lung cancer. Oncology 2000;59:291-5. [PubMed]
- Ichiki M, Rikimaru T, Gohara R, et al. Phase II study of irinotecan and ifosfamide in patients with advanced non-small cell lung cancer. Oncology 2003;64:306-11. [PubMed]
- D'Addario G, Früh M, Reck M, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21:v116-9. [PubMed]
- Lee KW, Kim YJ, Kim JH, et al. Two consecutive cases of platinum-refractory pulmonary pleomorphic carcinoma that showed dramatic responses to MAID (mesna, doxorubicin, ifosfamide and dacarbazine) chemotherapy. Jpn J Clin Oncol 2011;41:430-3. [PubMed]
- de Castro CG Jr, de Almeida SG, Gregianin LJ, et al. High-dose chemotherapy and autologous peripheral blood stem cell rescue in a patient with pleuropulmonary blastoma. J Pediatr Hematol Oncol 2003;25:78-81. [PubMed]
- Granata C, Gambini C, Carlini C, et al. Pleuropulmonary blastoma. Eur J Pediatr Surg 2001;11:271-3. [PubMed]
- Lobo-Sanahuja F, García I, Santamaría S, et al. Case report: pulmonary blastoma in children--response to chemotherapy. Med Pediatr Oncol 1996;26:196-200. [PubMed]
- Lindet C, Vanhuyse M, Thebaud E, et al. Pulmonary blastoma in adult: dramatic but transient response to doxorubicin plus ifosfamide. Acta Oncol 2011;50:156-7. [PubMed]


