Lung cancer screening guidelines: common ground and differences
Introduction
Lung cancer is the leading cause of cancer related mortality. There are approximately 159,260 lung cancer related deaths projected for 2014 in the USA, which accounts for one third of all cancer deaths (1). Despite significant advances in medical therapy, the overall 5-year survival rate for lung cancer has only increased from 11.4% in 1975 to 16.6% in 2009 as more than half of the cases are diagnosed at a metastatic stage with a 5-year survival of 3.9% (2). Only 15% cases are stage I at the time of diagnosis, which carries a higher 5-year survival rate of 53.5% (1). These rates give a rationale for lung cancer screening in high risk populations. For decades, tobacco control strategy has remained the cornerstone of lung cancer prevention strategies (3). Despite the reduction in the prevalence of smoking among adults from 43% to 18% (4) in 2010, since the release of US Surgeon General’s statement on impact of tobacco in 1964, the incidence of lung cancer has not been reduced proportionally. Smoking cessation does lowers tobacco attributable cancer risk but the risk never matches that of a non-smoker and a significant percentage of newly diagnosed lung cancers occur in former smokers (5). This pattern points to the evolving carcinogenic damage caused by tobacco smoke which continues despite cessation. Hence, combined efforts at smoking cessation and early screening seem prudent to tackle this ever increasing burden of disease. A decade has passed since the first randomized controlled trial (RCT) using low dose computed tomography (LDCT) was conducted by Garg et al. to assess the feasibility of early screening (6). After years of disappointing results from subsequent trials, a promising screening approach finally emerged with the National Lung Cancer Screening Trial (NLST), which is the most expensive trial ever conducted by National Cancer Institute (NCI) and spanned over a period of 9 years from 2002 to 2011. The trial reported a mortality reduction of 20% with LDCT screening as compared to chest X-ray (CXR) screening (7). NLST is the only completed, adequately powered study for lung cancer screening in a well-defined high risk population with concrete results so far. Since the results of NLST trial, data from NELSON (8) and I-ELCAP (9) projects have also come forth with results which further support the rationale behind lung cancer screening. These results formed the basis of the screening recommendations across almost all the major societies. After an comprehensive review of the literature and existing evidence, the U.S. Preventive Services Task Force (USPSTF) (10) along with the National Comprehensive Cancer Network® (NCCN®) (11), American Thoracic Society (ATS) (12), American Society of Clinical Oncology (ASCO) (13) and American Cancer Society (ACS) (14) have appraised the use of LDCT in early diagnosis of lung cancer and have endorsed a set of guidelines for its effective implementation. European Society for Medical Oncology (ESMO), however, recommends against lung cancer screening being offered to individual patients as a routine test. Patients requesting the screening test should be referred to a comprehensive programme with assured quality control, expertise in LDCT screening and infrastructure to ensure adequate follow up. The target patient population is same as the inclusion criteria for NLST trial (15).
Guidelines for screening of lung cancer: common ground and differences
After results of the NLST (7), I-ELCAP trial (9) and preliminary data from the NELSON trial (8), various societies released guidelines for lung cancer screening. Table 1 lists major society guidelines and pertinent follow up information wherever available. The target cohort in most of these guidelines mirrors the inclusion criteria of NSLT, which included adults between 55-74 years of age with at least 30 pack year history of smoking who were either current smokers or had quit smoking within the past 15 years. Only USPSTF has extended the upper limit of age eligible for screening till 80 years from 75 years (10). However, the consensus statement on withholding screening in individuals who quit smoking more than 15 years ago excludes a significant proportion of at-risk population, many of whom are healthy enough to undergo surgery for stage I lung cancer. Peto et al. have demonstrated that the risk of lung cancer decreases with smoking cessation at an earlier age, but it never returns to baseline. The cumulative risk of lung cancer by age 75 was 10%, 6%, 3% and 2% for men who quit smoking at ages 60, 50, 40, and 30 respectively (3).
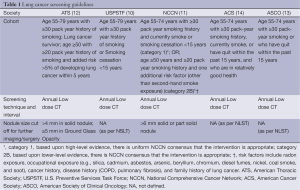
Full table
The mean age at diagnosis of lung cancer is 70 years as compared to 66 years for prostate cancer and 61 years for breast cancer (1). Consideration to include population with more than 30 pack year history of smoking under the age of 55 or individuals with a strong family history of cancer would help bridge this gap. It will also increase the rate of diagnosis at early stages of lung cancer. ATS has extended the benefits of screening to include lung cancer survivors, patients ≥50 years of age with ≥20 pack year history of smoking and an added risk of >5% to develop lung cancer within the next 5 years (12). NCCN® has a meticulous stratification of groups on the basis of age, smoking history and other risk factors (11). NCCN recommends screening in patients who meet the screening criteria of the NLST; this is a category 1 recommendation (based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate). NCCN also recommends (category 2B) screening in patients who are 50 years or older with a 20 or more pack year smoking history and at least one other risk factor (other than second hand smoke) such as radon exposure, occupational exposure, cancer history, family history of lung cancer, or history of lung disease (COPD or pulmonary fibrosis). A category 2B recommendation is based upon lower level evidence and there is NCCN consensus that the intervention is appropriate. Lower risk categories are acknowledged by NCCN but LDCT screening is not recommended due to narrower cost-benefit ratio and lack of compelling evidence.
Further research to develop more effective risk stratification tools to better define individuals at very high risk for inclusion into screening as well as to define lower risk groups which may not need the same frequency of screening is an important goal. Nevertheless, the use of current tools such as using self-reported tobacco use is an adequate tool to begin the national implementation of lung cancer screening for the United States.
Although the consensus on defining the appropriate target population for LDCT screening is very similar across the societies and for example all guidelines include provisions for informed decision making and inclusion of tobacco cessation services there is considerably more variation in regards to recommendations for follow up of a positive test result. ATS recommends follow up with 3-6 monthly imaging for a solid nodule between 4-8 mm size and ground glass opacity of more than 5 mm; and consideration for surgical removal of solid nodules more than 8 mm or nodules with rapid growth. NCCN recommends a threshold of 6 mm or more for solid and part solid nodules, and a threshold of 5 mm or more for ground glass opacities; further management depends on the size and type of the nodules and the growth pattern (11). Positron emission tomography (PET) scan can be considered for solid or part solid nodules greater than 8 mm (11). On the other hand, USPSTF, ASCO and ACS guidelines are not as detailed regarding work up of a positive result (10,13,14).
Most of these guidelines center around NLST trial results and the exploration of data from I-ELCAP and NELSON trials have been very conservatively applied. In part, related to the variability in management approach, there is still a considerable debate regarding the risks of screening. Key point is to use diagnostic work up requiring evidence of rapid nodule growth as this finding is indicative of clinically aggressive lung cancer. In this way we reduce false positive and reduce the possibility of overdiagnosis.
Overdiagnosis with LDCT annual screening
Overdiagnosis bias refers to detection of a cancer which, otherwise would never have become clinically apparent in a screening subjects’ lifetime or does not behave in a lethal fashion (16). It has always been an important concern while considering the benefit of screening. The distinction between false positive rate and overdiagnosis should be acknowledged while interpreting the data. Observational studies preceding NLST trial have estimated the extent of overdiagnosis between 13% and 27% with LDCT screening (17,18). This rate was calculated to be somewhere around 18% after 6.5 years of follow up in NSLT trial (19). However, this estimate is likely to be premature and it is more than likely that with a longer follow up period, the reported incidence of overdiagnosis will decrease owing to longer natural history of some LDCT detected cancers. For example there was an initial concern of overdiagnosis with CXR screening in the prostate, lung, colorectal and ovarian cancer screening trial (PLCO trial). A recent report of the PLCO trial found that the cumulative incidence of lung cancer after long term follow-up was similar in both the CXR and the control arm in high risk population (relative risk, 1.00; 95% confidence interval, 0.88 to 1.13) (20). This new finding reduces the concern for overdiagnosis to be a major confounder in evaluating the benefit of lung cancer screening. Data from California lung cancer registry also supports that degree of overdiagnosis is unlikely to be a major factor while defining the mortality benefit of lung cancer screening (21). Although overdiagnosis is an inevitable bias in screening studies, it can be mitigated with the advent of improving imaging modalities and precise definition of “positive result”. At the same time, focus should be placed on minimizing risks with diagnostic and surgical interventions to develop a highly valuable and reliable screening service.
Stepping stones to adoption of LDCT as a screening tool
LDCT refers to using 10-30% of the total radiation dose used in a standard non contrast CT scan. NLST used multi-detector CT scanners with an average estimated effective dose of 1.5 mSv average as compared to 5-7 mSv for standard CT (22). There has been a significant debate over adoption of LDCT as a screening tool. One of the major concerns is the risk of radiation induced cancer arising from LDCT itself and from subsequent imaging to work up the positive results. Regarding this issue, there was an important study that suggested that the risk of medical radiation exposure is considerably lower than the benefit of screening. Using the BEIR VII risk estimates, in an hypothetical screening scenario for an individual undergoing annual LDCT examination from age 55 to 74, the lifetime attributable risk (LAR) of lung cancer mortality resulting from radiation exposure is estimated to be 0.07% for males and 0.14% for females (23). To put this information in context, radiation exposure from natural and manmade sources can reach as high as 6.2 mSv per year and airline crew members are exposed to radiation levels as high as 2-6 mSv per year (24).
Since NLST, there have been continued improvements in LDCT technology and virtually all CT scanners in the United States can obtain LDCT scans at the doses used in the NLST or even lower. Ultra low dose CT scanners (ULDCT) techniques have been developed which deliver excellent images with less than 1 mSv exposure, which is comparable to radiation exposure by CXR. The efficacy of ULDCT was assessed in a study of 52 patients against traditional LDCT. ULDCT was found to have a true positive factor of 0.944 for nodules >4 mm in size, which is the current cut-off for reporting a positive result in screening cohort (25). Hence, with the continued technological advancements we can hope to achieve the same diagnostic accuracy with the least possible radiation exposure. These studies have been conducted in a population with normal BMI and the data needs to be extrapolated and validated in patients with higher BMIs as they comprise approximately more than one-third of current population of the USA (26).
Opportunities for rapid learning
NLST initially showed a 20% mortality benefit with LDCT screening as compared with CXR after three rounds of screening. Refinement of the approach to screening can potentially further improve this result. For example a re-analysis of NLST outcomes was conducted using an eligibility risk model constructed from PLCO case outcomes showed that they could define a higher risk cohort to use for the LDCT screening process (27). Two recent reports have suggested that sustained annual screening may reduce lung cancer mortality between 40% and 60% under different screening scenarios from analysis of the long term results of the New York Early Lung Cancer Action Project (NY-ELCAP) data (28,29). The benefits of LDCT screening are also promising from analysis of the preliminary data from NELSON trial as it show better stage I cancer detection rate compared to the NLST (Table 2), despite using different criteria for interpretation and diagnostic work up of a positive result (30). This demonstrates the scope for improvement in LDCT screening and the process of developing a good screening model.

Full table
Novel and promising principles for improving detection and diagnostic work up a nodule have been developed and inculcated into trial protocols since the NLST trial. NLST is a valuable data resource allowing for improvement in screening process and comparative interpretation of the same. Rapid refinement of CT scan resolution and development of newer techniques such as ULDCT have resulted in early and reliable detection of stage I smaller primary lung cancer. Since most of the pulmonary nodules less than 1 cm in diameter are benign in setting of a screening test (31,32), various concepts of volumetric analysis of nodule detection and restricting diagnostic work up to nodules which show significant growth over time have been tested (33). NELSON study design used this interval-growth diagnostic work up resulting in a diagnostic sensitivity of 95% and a specificity of 99% for LDCT. The rate of invasive diagnostic work up was 12% in NELSON (8). This interval-growth criterion for suspicious nodules was also applied in a cohort of 4,700 screening patients and only 3% of the patients underwent invasive diagnostic work up and the rate of false positive detection was 0.42% (34). Another approach is to change the threshold of nodule size for detection which was included in I-ELCAP trial. Reducing the nodule size threshold from 4-5 to 7-8 mm significantly reduced the frequency of “false positive” lung cancers while maintaining the diagnostic accuracy (35). However, in raising the threshold for nodule size to 8 mm there would have been a delay of 9 months in 6% of the patients diagnosed with stage I lung cancer within 1 year of baseline screening in I-ELCAP.
New information also suggests it is useful to consider nodule characteristics such as ground glass, solid, non-solid and part solid during evaluation. It is known that only 20% of pure and 40% of part solid GGOs gradually grow over time with a doubling time of 600-900 and 300-450 days respectively (36). Fleischer society further recommends no additional surveillance work up for pure GGOs 5 mm diameter or less (37). NCCN has already incorporated the results from this study and has stratified the nature and intensity of follow up and diagnostic work up based on nodule size and interval growth (11). Figure 1 shows the NCCN clinical practice guidelines in oncology (NCCN Guidelines®) for lung cancer screening, for work up of solid or part solid nodule found during screening evaluation.
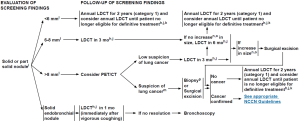
At the time of NLST, surgical care protocols were not developed and minimally invasive surgery was still in the nascent stages. Minimally invasive technique involving sub lobar resection was analyzed in a retrospective review of 347 thoracic resections and long term (10 years) results of sublobar resection were equivalent to lobectomy in clinical stage IA cancers (38). These results favor detection of stage I cancers with screening and provide added benefit of preserving large amount of well-functioning tissue, hence lowering the post-operative morbidity.
All these advancements require an infrastructure to include rapid learning and implementation of the same to structure a highly efficacious and cost effective algorithm in lung cancer screening and its implementation as a public screening service. Using published approaches from the I-ELCAP experience, actuarial simulation models have reported this implementation to be cost effective. A cost benefit analysis in 2012 estimated the cost of screening for lung cancer to be $247 per person screened per annum assuming that 75% of screenings were repeat testing, which is in concordance with the data of a large collaborative study of low-dose spiral CT screening in population ages 50-55 (9,39). As well, in the setting of a commercial insurer, the incremental cost of providing LDCT service to a routine full medical coverage plan was estimated to be around $0.76 per member per month. By comparison, this cost was significantly lower than the insurer cost for breast, colorectal, or cervical cancer which was $2.50, $0.95, and $1.10 respectively (39). An older patient-level micro simulation study showed that annual screening of current and former smokers aged 50 to 74 years would cost between $154,000 and $207,000 (2012 USD) per quality-adjusted life year saved as compared to no intervention (40). However, Pyenson and co-workers using estimates of cost and outcomes from best current practice have predicted that with annual LDCT screening, 985,284 quality adjusted life years could be saved over the next 15 years (41).
NCCN (11) has emerged as a useful source of frequently updated lung cancer screening process information. They recommend the use of state-of-the-art infrastructure comprising sophisticated multi-detector CT scanners, analytical software, physicists and radiologists to perform testing at acceptable radiation exposures and use of standardized terminology for interpretation and appropriate guidelines to report the results. It also requires a reliable system to communicate with the screening subject and primary care physicians to ensure the tracking of screened individuals and documenting outcomes.
Implementation of LDCT screening can prove to act as a smoking cessation intervention itself. Twenty three percent of active smokers reported quitting after first annual round of screening in ELCAP trial (42), as against the background quit rate in general population of 4%. Further, with the addition of smoking cessation to that screening process, the cost utility ratio of quality adjusted life years could be reduced from $28,240 to $16,198 per life year gained. Hence, apart from reducing medical costs, inclusion of smoking cessation interventions will help reduce mortality and morbidity more than the screening alone.
Conclusions
Despite promising results, the adoption of lung cancer screening has been slow. We now know that LDCT screening reduces mortality by allowing the more frequent diagnosis of lung cancer at an early stage. Furthermore, LDCT screening along with smoking cessation interventions is cost effective. LDCT as a screening modality has several robust features. It is painless, quick and easily available. The risk of radiation exposure associated with annual screening LDCT is often overstated and overestimated and is in fact low. This amount of radiation exposure in older, heavily tobacco-exposed populations, it should not deter the high risk populations from seeking screening testing. Overdiagnosis bias can be mitigated by the inclusion of case selection using serial LDCT scans to restrict diagnostic work-ups to individuals that demonstrate rapid pulmonary nodule growth. With the marked improvement in diagnostic evaluation of pulmonary nodule, more tailored and minimally invasive surgical techniques and improvements in LDCT technology, the case for adoption of lung cancer screening as a public health policy is stronger than ever. Under the Affordable Care Act all commercial insurers will provide LDCT to their beneficiaries and from a health equity perspective, a strong case exists for CMS to provide this cancer screening service soon as well. Thoughtful implementation of a high quality new lung cancer screening service along with necessary measures for tracking outcomes is a national matter of urgent priority.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [PubMed]
- National Cancer Institute. Surveillance epidemiology and end results: SEER stat fact sheets: lung and bronchus. Available online: http://seer.cancer.gov/statfacts/html/lungb.html#survival
- Peto R, Darby S, Deo H, et al. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ 2000;321:323-9. [PubMed]
- The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. Available online: http://www.surgeongeneral.gov/library/reports/50-years-of-progress/
- Tong L, Spitz MR, Fueger JJ, et al. Lung carcinoma in former smokers. Cancer 1996;78:1004-10. [PubMed]
- Garg K, Keith RL, Byers T, et al. Randomized controlled trial with low-dose spiral CT for lung cancer screening: feasibility study and preliminary results. Radiology 2002;225:506-10. [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409.[PubMed]
- Ru Zhao Y, Xie X, de Koning HJ, et al. NELSON lung cancer screening study. Cancer Imaging 2011;11 Spec No A:S79-84.
- International Early Lung Cancer Action Program Investigators, Henschke CI, Yankelevitz DF, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763-71. [PubMed]
- Humphrey L, Deffebach M, Pappas M, et al. Screening for Lung Cancer: Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Available online: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0060145/
- Reproduced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Lung Cancer Screening V.2.2014.© 2014 National Comprehensive Cancer Network, Inc.All rights reserved.The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of the NCCN.To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org.NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, NCCN GUIDELINES®, and all other NCCN Content are trademarks owned by the National Comprehensive Cancer Network, Inc. Available online: http://www.nccn.org/patients/guidelines/lung_screening/index.html
- Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg 2012;144:33-8. [PubMed]
- The role of CT screening for Lung Cancer in clinical practice. The evidence based practice guideline of the American College of Chest Physicians and the American Society for Clinical Oncology. Available online: http://www.asco.org/quality-guidelines/role-ct-screening-lung-cancer-clinical-practice-evidence-based-practice-guideline
- Wender R, Fontham ET, Barrera E Jr, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin 2013;63:107-17. [PubMed]
- Vansteenkiste J, Crinò L, Dooms C, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol 2014. [Epub ahead of print].
- Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst 2010;102:605-13. [PubMed]
- Lindell RM, Hartman TE, Swensen SJ, et al. Five-year lung cancer screening experience: CT appearance, growth rate, location, and histologic features of 61 lung cancers. Radiology 2007;242:555-62. [PubMed]
- Sone S, Nakayama T, Honda T, et al. Long-term follow-up study of a population-based 1996-1998 mass screening programme for lung cancer using mobile low-dose spiral computed tomography. Lung Cancer 2007;58:329-41. [PubMed]
- Patz EF Jr, Pinsky P, Gatsonis C, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014;174:269-74. [PubMed]
- Marcus PM, Bergstralh EJ, Zweig MH, et al. Extended lung cancer incidence follow-up in the Mayo Lung Project and overdiagnosis. J Natl Cancer Inst 2006;98:748-56. [PubMed]
- Raz DJ, Zell JA, Ou SH, et al. Natural history of stage I non-small cell lung cancer: implications for early detection. Chest 2007;132:193-9. [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Berg CD, et al. The National Lung Screening Trial: overview and study design. Radiology 2011;258:243-53. [PubMed]
- Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Available online: http://www.nap.edu/openbook.php?isbn=030909156X
- dos Santos Silva I, De Stavola B, Pizzi C, et al. Cancer incidence in professional flight crew and air traffic control officers: disentangling the effect of occupational versus lifestyle exposures. Int J Cancer 2013;132:374-84. [PubMed]
- Yamada Y, Jinzaki M, Tanami Y, et al. Model-based iterative reconstruction technique for ultralow-dose computed tomography of the lung: a pilot study. Invest Radiol 2012;47:482-9. [PubMed]
- Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010;303:235-41. [PubMed]
- Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med 2013;368:728-36. [PubMed]
- Foy M, Yip R, Chen X, et al. Modeling the mortality reduction due to computed tomography screening for lung cancer. Cancer 2011;117:2703-8. [PubMed]
- Henschke CI, Boffetta P, Gorlova O, et al. Assessment of lung-cancer mortality reduction from CT Screening. Lung Cancer 2011;71:328-32. [PubMed]
- van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361:2221-9. [PubMed]
- Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354:99-105. [PubMed]
- Swensen SJ, Jett JR, Sloan JA, et al. Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med 2002;165:508-13. [PubMed]
- Yankelevitz DF, Reeves AP, Kostis WJ, et al. Small pulmonary nodules: volumetrically determined growth rates based on CT evaluation. Radiology 2000;217:251-6. [PubMed]
- Wagnetz U, Menezes RJ, Boerner S, et al. CT screening for lung cancer: implication of lung biopsy recommendations. AJR Am J Roentgenol 2012;198:351-8. [PubMed]
- Henschke CI, Yip R, Yankelevitz DF, et al. Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med 2013;158:246-52. [PubMed]
- Mun M, Kohno T. Efficacy of thoracoscopic resection for multifocal bronchioloalveolar carcinoma showing pure ground-glass opacities of 20 mm or less in diameter. J Thorac Cardiovasc Surg 2007;134:877-82. [PubMed]
- Kim H, Park CM, Koh JM, et al. Pulmonary subsolid nodules: what radiologists need to know about the imaging features and management strategy. Diagn Interv Radiol 2014;20:47-57. [PubMed]
- Sone S, Nakayama T, Honda T, et al. Long-term follow-up study of a population-based 1996-1998 mass screening programme for lung cancer using mobile low-dose spiral computed tomography. Lung Cancer 2007;58:329-41. [PubMed]
- Pyenson BS, Sander MS, Jiang Y, et al. An actuarial analysis shows that offering lung cancer screening as an insurance benefit would save lives at relatively low cost. Health Aff (Millwood) 2012;31:770-9. [PubMed]
- McMahon PM, Kong CY, Bouzan C, et al. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol 2011;6:1841-8. [PubMed]
- Villanti AC, Jiang Y, Abrams DB, et al. A cost-utility analysis of lung cancer screening and the additional benefits of incorporating smoking cessation interventions. PLoS One 2013;8:e71379. [PubMed]
- Ostroff JS, Buckshee N, Mancuso CA, et al. Smoking cessation following CT screening for early detection of lung cancer. Prev Med 2001;33:613-21. [PubMed]


