Adjuvant chemotherapy in non-small cell lung cancer: state-of-the-art
Introduction
Surgery remains the basic treatment for patients with localized non-small cell lung cancer (NSCLC). Nonetheless, even after an apparently complete resection procedure the risk of recurrence remains substantial. The benefit of adjuvant chemotherapy (AC) was not demonstrated until one decade ago with the repot of trial exploring the value of active cisplatin combinations along with optimal supportive care measures.
Pathological stage is the single most relevant prognostic factor for recurrence and death after NSCLC surgery. For patients with pathological stage II the 5-year survival rate after surgery alone is under 50% (stage IIA 46%, and IIB 36%) and it drops as low as to 24% for stage IIIA (1). Significant efforts have been made to refine prognostic information with molecular markers (such as K-ras mutations and ERCC-1 expression) (2,3) or gene expression signatures, but up to the present they remain investigational and need to be confirmed in prospective trials which are currently active (4).
AC is currently recommended for patients with pathologic stages II and III after surgery with curative intent. It is not for stage IA and its role in stage IB is limited and based on lower evidence. Theoretical considerations make postoperative chemotherapy appealing: i.e., the percentage of relapse in these tumors is high, and most of the relapses are systemic (lung, CNS, bone, adrenal and liver being the commonly involved organs) as well as the earlier proof of benefit in other common primary tumors such as breast or colorectal carcinomas. However it took a longer time to demonstrate its benefit in NSCLC.
Trials conducted prior to 2000 were small and had several methodological flaws that did not permit to reach any conclusion and lead to a nihilistic attitude. A meta-analysis published in 1995 (5) showed that alkylating-based regimens were detrimental in terms of survival (a 15% increased risk of death existed). On the contrary, for eight trials that used cisplatin-based regimens a 5% absolute benefit in 5-year survival was found (albeit without statistical significance due to small size). This finding gave new thrust to AC and several new trials with Cisplatin combinations were started.
Besides of the intrinsic effectiveness of chemotherapy a second factor had to be taken into consideration. Compliance of patients with the scheduled chemotherapy was poor. A thoracotomy is a significant surgical procedure and requires some time to recover. Besides, by the time when those trials were conducted supportive measures (antiemetic therapy and colony-stimulating stimulating factors mainly) were suboptimal.
Modern trials with AC
After the results of the above mentioned meta-analysis were published, several trials comparing cisplatin-based schemes vs. observation were conducted. We will review them and a brief summary of their characteristics is also presented in Table 1.

Full table
- The Adjuvant Lung Project (ALPI) was the first reported (and the last negative) of these trials (6). It was a large trial with 1,209 patients enrolled. Stages I,II and IIIA were included and randomization arms were mitomycin, vindesine and cisplatin (MIC) for three cycles or no treatment. This trials found no differences between arms in survival [HR 0.96 (95% CI, 0.81-1.13, P=0.59)] or progression-free survival [HR 0.89 (95% CI, 0.76-1.03, P=0.13)]. Only 69% of the patients received the three planned courses and grade IV neutropenia occurred in 12% of patients.
- A second negative trial was published (8). However, this trial was smaller (381 patients) and probably did not meet quality standards to be taken into account: 3% of the patients received pre-operative chemotherapy, 5% did not reached complete resections, multiple chemotherapy schemes were allowed and 13% did not even started scheduled therapy. No benefit in survival was found [HR 1.02 (95% CI, 0.77-1.35, P=0.90)] and 30% of the patients had grades III,IV toxicity.
- The, up to now, larger evidence for AC in this setting comes from the International Adjuvant Lung Cancer Trial (IALT) (7). It included 1,867 patients with resected stages I to IIIA. Of them, 932 patients were allocated to the chemotherapy arm and 74% received a Cisplatin dose of 240 mg/m2 or more. Chemotherapy included three or four courses of Cisplatin based chemotherapy along with either etoposide (56.5% of the patients) vinorelbine (26.8%) vinblastine (11.0%) or vindesine (5.8%). AC arm had a significant higher survival rate than the observation arm [44.5% vs. 40.4% at 5 years; HR 0.86 (95% CI, 0.76-0.98, P<0.03)]. Progression-free survival was also superior [39.4 vs. 34.3 at 5 years [HR 0.83 (95% CI, 0.74-0.94, P<0.003)]. There were a 0.8% of chemotherapy-related deaths.
- JBR.10 (10) was a trial conducted in Canada and USA. A total of 482 patients with resected NSCLC were included. Chemotherapy was vinorelbine 25 mg/m2 weekly for 16 weeks plus cisplatin 50 mg/m2 on days 1 and 8, for four courses. Forty-five percent of the patients were stage IB and 55% stage II (excluding T3N0 patients). Chemotherapy significantly prolonged progression-free survival as compared with observation [HR 0.60 (95% CI, 0.45-0.79, P<0.001)] and survival at 5 years [69% vs. 54% (HR 0.78)]. Subgroup analysis showed no survival benefit for stage IB patients (P=0.79) whilst for stage II median survival was 80 months for the chemotherapy arm vs. 41 months for the observation arm [HR 0.59 (95% CI, 0.42-0.85, P=0.004)]. In this trial the most common toxicity was hematologic (with 7% of febrile neutropenia) and there were two treatment-related deaths. A study of quality of life (QoL) was conducted in a subset of 359 patients. Chemotherapy was associated with a transient worsening in QoL due to nausea, vomiting and fatigue but returned to baseline by 9 months (except for neurotoxicity).
- The trial conducted by the Adjuvant Navelbine International Trialist Association (ANITA) (11) also randomized patients with stage Ib, II or IIIa to a cisplatin-vinorelbine combination (Vinorelbine 30 mg/m2 weekly for 16 weeks plus cisplatin 100 mg/m2 day 1 every 4 weeks, 4 courses scheduled) or observation. Chemotherapy significantly improved median survival (65.7 vs. 43.7 months) with an 8.6% absolute benefit at 5 years [HR 0.80 (95% CI, 0.66-0.96, P=0.02)]. Neutropenia was the main toxicity (9% febrile neutropenia) with 2% toxic deaths.
- One more trial has to be mentioned (9) although with some caveats in mind. CALGB 9633 was relatively small (344 patients) and was the only large trial which used carboplatin AUC 6 (instead of cisplatin) along with paclitaxel 200 mg/m2. Four courses given every 3 weeks were scheduled. It is also remarkable that only patients with resected stage IB disease were included. In a preliminary report with 34 months median follow-up AC was associated with a significant improvement in progression-free and overall survival. However, with a longer median follow-up of 74 months differences in survival were non-significant [HR 0.83 (95% CI, 0.64-1.08, P=0.12)]. All these factors (insufficient statistical power, early stop, carboplatin use, stage IB) may have influenced the results. Subsequently, an exploratory analysis showed benefit for patients whose tumors were 4 cm in diameter or larger (HR 0.69).
Meta-analysis
Given the heterogeneity of the described trials, several meta-analyses have been reported to combine the results. The Lung Adjuvant Cisplatin Evaluation (LACE) meta-analysis is the most important (12). Individual patient data were collected and pooled from the five largest trials of cisplatin-based chemotherapy which included 4,584 patients. With a median follow-up of 5.2 years the overall HR for death was 0.89 (95% CI, 0.82-0.96, P=0.005) corresponding to a 5-year absolute benefit of 5.4% derived from chemotherapy. No heterogeneity of chemotherapy effect was found among trials. The benefit varied with stage (P=0.04) HR being for stage IA 1.4, IB 0.93, II 0.83 and III 0.83. The drug given with cisplatin did not modify the effect of chemotherapy (P=0.11): vinorelbine was higher (0.80), etoposide or other vinca alkaloid 0.92, the rest 0.97. The effect of chemotherapy was greater in patients with better performance status and no influence of other variables (sex, age, histology, type of surgery, planned radiotherapy (RT) of planned total dose of cisplatin) was found.
Other meta-analyses have been reported later, confirming these results. A subsequent publication from the group of earlier 1995 meta-analysis (13) including these and older trials (8,447 patients overall) confirmed the benefit of the addition of chemotherapy. The benefit in this analysis translated into a HR 0.86 (95% CI, 0.81-0.92, P<0.0001) for survival (4% absolute benefit at 5 years (from 60% to 64%). In this case the role of RT was also analyzed in 13 trials and 2,660 patients. Again a 4% absolute benefit in survival at 5-year (from 29% to 33%) was found for chemotherapy-surgery-RT vs. surgery plus RT. Little variation in effect according to the type of chemotherapy or patient subgroups was found either. A third, more recent meta-analysis has been reported (14) and the results were similar: HR for death 0.76 and 5% reduction in 2-year mortality.
Whether the effect of AC maintains over time has been object of controversy. On one hand, follow-up results of the IALT trial were reported (15) and after 5 years of follow-up the benefit for death and relapse showed a trend to decline (but curve of chemotherapy kept always over the observation arm). This was not due to a reduced effect on the anti-tumor effect (both local recurrences and distant metastasis were reduced during the whole period for the chemotherapy arm, second primaries being similar in both groups) but to an increase in non-tumor related deaths (which could be a sign of delayed toxicity of chemotherapy) as HR was 1.34 (P=0.06). On the other, a longer follow-up of JBR10 trial showed a maintained benefit in survival (HR 0.78, P=0.04) and tumor-specific disease-free survival (HR 0.73, P=0.03) at least for patients with nodal involvement (16).
Anyway, this issue remarks the value of a proper selection of the candidates to AC (poorer performance status could be associated to a decreased benefit) and the continuing research in this field to preserve the survival benefit on the long term.
Toxicity
Side-effects of CT have been a concern since the first trials, when compliance was suboptimal most of the times. Table 2 shows the most relevant grades III,IV side effects in the above mentioned trials.
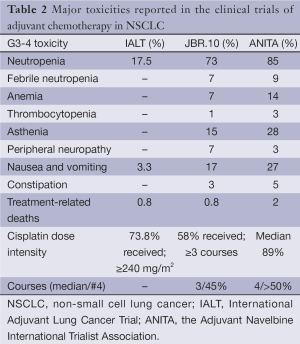
Full table
Notwithstanding, it must be remarked that toxicity tends to be transient and solved a few months after adjuvant therapy has been finished. This was exemplified by Bezjak (17) with data from trial JBR-10. He showed that with the exception of sensory neuropathy and hearing loss, the rest of side effects were recovered and quality of live returned to the baseline by 9 months after treatment.
Potential damage of pulmonary functional activity has been another fear after AC in these patients. However, a recent paper (18) noted that no decrease in pulmonary function was seen after chemotherapy in 132 patients included in a clinical trial.
Elderly patients
Elderly patients represent a relevant population as a significant percentage of NSCLC is currently diagnosed in patients over 70 years old. Current data do not support sparing AC based in age only as potential benefits of the therapy are maintained in them.
It has to be noticed that in the mentioned clinical trials patients over 75 years old represented less than 10% of the enrolled population. This may represent a selection bias and besides reduces the external validity of the data. However, the LACE meta-analysis addressed this specific issue and found no differences in efficacy or toxicity in elderly patients. Interestingly, CT dose and number of courses do were reduced indeed suggesting that those patients trend to be treated in practice with lower doses of standard schemes to preserve efficacy and tolerability.
This trend in clinical practice has also been mentioned elsewhere (19). In a Canadian report of 3,354 patients with radical surgery for NSCLC only 1,830 (55%) were referred to a medical oncologist for consultation. Patients over 70 years were less likely referred [odds ratio (OR) =0.4; P<0.001]. Amongst those who were referred, older patients (60-69-year-old, OR =0.4; >70-year-old, OR =0.1), patients with greater comorbidity (Charlson comorbidity index 3+ OR =0.5) or with longer postoperative stay (>7 days, OR =0.7) were less likely to receive AC.
Other issues concerning AC
Related with the necessity of adequate recovery from prior surgery before starting AC stands the proper timing to initiate this treatment. The optimal time of initiation is unknown but a period up to 8 weeks after surgery is deemed to be appropriate by analogy with breast and colon cancer AC. Delays beyond this point could be associated with inferior cancer-specific survival. No prospective data have been reported, but a retrospective analysis of 1,032 cases of patients receiving AC has been published (20). In clinical practice the authors found a trend between delayed AC (starting more than 8 weeks after surgery) and longer post-operative hospital stay (P=0.054) or readmissions (P=0.056). This delay was no associated to inferior overall survival (OR =1.0). In fact one third of the patients started AC after 10 weeks from surgery.
It was already mentioned that CALGB trial (9) was negative for N0 patients. A post-hoc retrospective analysis found that patients whose tumors were at least 4 cm in diameter benefited from AC. This has been confirmed by others (21) and it is considered adequate to treat with AC patients with tumor greater than 4 cm (particularly if they are poorly differentiated and showed vascular invasion) in selected patients.
Histology is a prognostic factor in advanced disease. However, it has not been shown a major effect in early stages at least with the chemotherapy schemes we have available (22).
Other appealing issue in the field of AC is the use of targeted therapy. The use of inhibitors of the tyrosine-kinase of the epidermal growth factor receptor has been standard in advanced disease in the past years and the expansion of its utility in the adjuvant setting is tempting. However, right now its benefit has not been proven yet. We only have one reported trial comparing and EGFR inhibitor [gefitinib (G)] versus placebo (23). This trial was prematurely closed (with 503 out of 1,242 planned patients enrolled) because of the results of other trials with G (mainly S0023, an intergroup trial assessing the value of adjuvant G after chemo-RT for locally-advanced NSCLC). In the setting of AC and with 4.7 years of follow-up no differences existed between G and placebo (HR 1.24 for overall survival and 1.22 for disease-free survival). Of note, no differences existed for EGFR-mutant patients either, albeit only 15 of them were EGFR-positive. Chinese trials are currently specifically studying inhibitors in the EGFR-positive population: both G vs. placebo (NCT01405079) and Erlotinib vs. Cisplatin-Vinorelbine (NCT01410214).
Some other new and targeted agents are currently under investigation (24). Bevacizumab is one of them. NCT00324805 is and North American Intergroup trial recruiting patients with stage IB to IIIA that are randomized to either Cisplatin-based chemotherapy (with either gemcitabine vinorelbine docetaxel or pemetrexed) with or without Bevacizumab (25). Endostatin is an angiostatic protein that has been evaluated (26) but results are not yet available. Immunotherapy is also of interest at this point. MAGE A3 is an antigen currently under evaluation and shows some hint of potential activity in this setting (27).
RT has been considered not to be useful (or even detrimental) in the adjuvant setting since the report of the PORT meta-analysis. However there are some retrospective data indicating that at least in some N2 patients treated with AC based on Cisplatin and Vinorelbine it could play a role (28) and merit further investigation.
Guidelines from scientific societies
As a consequence of its reported activity and the proven benefit from large randomized clinical trials AC has been progressively included into recommendations and guidelines by most scientific societies. Published as early as 2007 ASCO Guidelines endorsed the use of AC for NSCLC in patients with nodal involvement (either N1 or N2 levels) (29).
Also NCCN shares this view and recommend AC in the same setting (30). No uniform recommendations according the optimal chemotherapy regimen exists. As a general consensus a combination of Cisplatin plus a second-generation chemotherapy drug is endorsed. NCCN Guideline mentions Cisplatin (50 to 100 mg/m2) along with Vinorelbine (either 25 to 30 mg/m2 days 1 and 8 on each course or on a weekly basis). Cisplatin combinations with Etoposide, Gemcitabine, Docetaxel and Pemetrexed (the latter for adenocarcinoma only) are also selected (31). In our view, Vinorelbine is probably the drug for which more robust evidence exists to be the companion with Cisplatin (32).
More controversial may be substituting cisplatin with carboplatin in this setting, as the only reported trial was negative (9). Nonetheless, some authors would accept a carboplatin-paclitaxel regimen as a valid option for those patients with comorbidities or not able to tolerate cisplatin (33).
American College of Chest Physicians (ACCP) is one more example of organization developing evidence-based medicine tools that includes AC in its Guidelines (34,35) as well as the Spanish Society for Medical Oncology (SEOM) (36). All of them mention that optimal therapy should include four courses of such chemotherapy.
Summary and recommendations
The current clinical scenario in which AC has become a standard in NSCLC therapy has been described. Clinical evidence supports its use in terms that are comparable with breast or colon cancer. Nonetheless, as also has been mentioned, there are a lot of questions that remain unsolved. Patient’s selection is key in order to preserve the survival benefit in a clinical setting in which many variables may have an influence in the final outcome: performance status, comorbidities, pathological stage, type and complications of surgery, chemotherapy scheme, prognostic molecular factors, the role of RT and targeted drugs.
Clinical research must be encouraged to find responses and improve chances for the survival of these patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [PubMed]
- Shepherd FA, Domerg C, Hainaut P, et al. Pooled analysis of the prognostic and predictive effects of KRAS mutation status and KRAS mutation subtype in early-stage resected non-small-cell lung cancer in four trials of adjuvant chemotherapy. J Clin Oncol 2013;31:2173-81. [PubMed]
- Soria JC, Barlesi F, Besse B, et al. Results of the prospective, randomized, and customized NSCLC adjuvant phase II trial (IFCT-0801, TASTE trial) from the French Collaborative Intergroup. J Clin Oncol 2013;31(suppl; abstr 7505).
- Kratz JR, He J, Van Den Eeden SK, et al. A practical molecular assay to predict survival in resected non-squamous, non-small-cell lung cancer: development and international validation studies. Lancet 2012;379:823-32. [PubMed]
- Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 1995;311:899-909. [PubMed]
- Scagliotti GV, Fossati R, Torri V, et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell Lung cancer. J Natl Cancer Inst 2003;95:1453-61. [PubMed]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60. [PubMed]
- Waller D, Peake MD, Stephens RJ, et al. Chemotherapy for patients with non-small cell lung cancer: the surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg 2004;26:173-82. [PubMed]
- Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043-51. [PubMed]
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589-97. [PubMed]
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719-27. [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [PubMed]
- NSCLC Meta-analyses Collaborative Group, Arriagada R, Auperin A, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet 2010;375:1267-77. [PubMed]
- Zhong C, Liu H, Jiang L, et al. Chemotherapy plus best supportive care versus best supportive care in patients with non-small cell lung cancer: a meta-analysis of randomized controlled trials. PLoS One 2013;8:e58466. [PubMed]
- Arriagada R, Dunant A, Pignon JP, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol 2010;28:35-42. [PubMed]
- Butts CA, Ding K, Seymour L, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol 2010;28:29-34. [PubMed]
- Bezjak A, Lee CW, Ding K, et al. Quality-of-life outcomes for adjuvant chemotherapy in early-stage non-small-cell lung cancer: results from a randomized trial, JBR.10. J Clin Oncol 2008;26:5052-9. [PubMed]
- Kreuter M, Vansteenkiste J, Herth FJ, et al. Impact and safety of adjuvant chemotherapy on pulmonary function in early stage non-small cell lung cancer. Respiration 2014;87:204-10. [PubMed]
- Kankesan J, Shepherd FA, Peng Y, et al. Factors associated with referral to medical oncology and subsequent use of adjuvant chemotherapy for non-small-cell lung cancer: a population-based study. Curr Oncol 2013;20:30-7. [PubMed]
- Booth CM, Shepherd FA, Peng Y, et al. Time to adjuvant chemotherapy and survival in non-small cell lung cancer: a population-based study. Cancer 2013;119:1243-50. [PubMed]
- Cuffe S, Bourredjem A, Graziano S, et al. A pooled exploratory analysis of the effect of tumor size and KRAS mutations on survival benefit from adjuvant platinum-based chemotherapy in node-negative non-small cell lung cancer. J Thorac Oncol 2012;7:963-72. [PubMed]
- Bennouna J, Senellart H, Hiret S, et al. Impact of histology on survival of resected non-small cell lung cancer (NSCLC) receiving adjuvant chemotherapy: subgroup analysis of the adjuvant vinorelbine (NVB) cisplatin (CDDP) versus observation in the ANITA trial. Lung Cancer 2011;74:30-4. [PubMed]
- Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 2013;31:3320-6. [PubMed]
- Simon GR, Somaiah N. A tabulated summary of targeted and biologic therapies for non-small-cell lung cancer. Clin Lung Cancer 2014;15:21-51. [PubMed]
- Available online: http://clinicaltrials.gov/ct2/show/NCT00324805?term=NCT00324805&rank=1
- Available online: http://clinicaltrials.gov/ct2/show/NCT01124253?term=adjuvant+lung+endostar&rank=2
- Cuppens K, Vansteenkiste J. Vaccination therapy for non-small-cell lung cancer. Curr Opin Oncol. 2014;26:165-70. [PubMed]
- Douillard JY, Rosell R, De Lena M, et al. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int J Radiat Oncol Biol Phys 2008;72:695-701. [PubMed]
- Pisters KM, Evans WK, Azzoli CG, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol 2007;25:5506-18. [PubMed]
- Available online: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site
- Simon GR, Manegold C, Barker SS, et al. Pemetrexed use in the adjuvant setting for completely resectable non-small-cell lung cancer. Clin Lung Cancer 2013;14:601-8. [PubMed]
- Douillard JY, Tribodet H, Aubert D, et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the Lung Adjuvant Cisplatin Evaluation. J Thorac Oncol 2010;5:220-8. [PubMed]
- Chang WJ, Sun JM, Lee JY, et al. A retrospective comparison of adjuvant chemotherapeutic regimens for non-small cell lung cancer (NSCLC): paclitaxel plus carboplatin versus vinorelbine plus cisplatin. Lung Cancer 2014;84:51-5. [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e314S-40S.
- Camps C, Felip E, García-Campelo R, et al. SEOM clinical guidelines for the treatment of non-small cell lung cancer (NSCLC) 2013. Clin Transl Oncol 2013;15:977-84. [PubMed]


