Circulating soluble intercellular adhesion molecule-1 in lung cancer: a systematic review
Introduction
Lung cancer is the leading cause of malignancy-related death worldwide. Despite advances in diagnostic and therapeutic techniques, the prognosis of lung cancer patients is still poor, with an overall 5-year survival rate of approximately 15% (1). The extremely poor prognosis associated with lung cancer is related to the difficulty of early diagnosis and high incidence of regional or distant metastasis. However, the interactions between tumor cells and the vascular endothelium is essential for tumor metastasis (2). And intercellular adhesion molecule-1 (ICAM-1) has been thought to play an important role in the specific steps of the metastatic process in malignant diseases (3,4).
ICAM-1, a member of the immunoglobulin supergene family, is a single-chain cell surface glycoprotein which is expressed constitutively at low levels on different types of cells (5). The molecular interaction between ICAM-1 and its ligand the leukocyte integrin lymphocyte function-associated antigen (LFA-1) is a crucial step for the transendothelial migration of leukocytes. Moreover, cytokine-induced expression of ICAM- 1 can render tumor cells more sensitive to monocyte- and T cell-mediated lysis (6,7). A soluble form of ICAM-1 (sICAM-1) was firstly identified in health volunteers' serum by Seth et al.(8). Although sICAM-1 is smaller than its membrane-bound form, its five immunoglobulin-like domains and its ability to bind with LFA-1 are conserved (9). In this way, sICAM-1 can bind to circulating cytotoxic lymphocytes, block the interaction between tumor cells and the APC or T lymphocytes, and thus allow tumors to escape immune recognition (10). Furthermore, Gho et al. (11,12) had reported that sICAM-1 apparently have the ability to promote angiogenesis and stimulate tumor cells growth. Elevated sICAM-1 levels have been reported in patients with a variety of malignancies, and it has been though to correlate with disease progression and tumor metastasis (10,13,14).
In lung cancer patients, several studies have reported the presence of increased levels of sICAM-1 and the relationship between its concentration and clinical outcome as well as clinico-pathological characteristics, including ECOG Performance status, gender, age, smoking history, histological type, tumor stage, and others (15-18). Although these subjects have been studied for over a decade, no consensus has been reached and some conflicting results have been reported from different studies. In this review, we shall summarize to the best of our ability the results of these studies on lung cancer and mainly focus on four aspects as follows: (I). the comparison of sICAM-1 levels between lung cancer patients and healthy controls, (II). the relationship between sICAM-1 levels and lung cancer patients' clinicopathological characteristics, (III). the changes of sICAM-1 levels during treatment courses, (IV). the prognostic and predictive implications of sICAM-1 levels in lung cancer.
Methods
Search Strategy and Study Selection
In order to review the literature about sICAM-1and lung cancer, we did a search on electronic databases (including PubMed, Web of Science, and Medline) with the terms "ICAM", "intercellular adhesion molecule", and "lung cancer", with the search limited to title or abstract. And an upper date limit of July 6, 2011 was applied; we used no lower date limit. We also screened the reference lists from identified primary studies to identify any studies which appeared to be appropriate for this review.
Studies eligible for inclusion in this review met the following criteria: (I) proven diagnosis of lung cancer in humans, (II) evaluate the circulating sICAM-1 levels, and (III) provide information about at least one of four above mentioned aspects. There was no special requirement for sample size or follow-up period. When the same author published multiple manuscripts and used overlapping patient cohorts, only the most recent report or the most complete one was included in this review. Reports in journals which were difficult to access were also excluded for detailed review. All the candidate articles were independently read and checked for inclusion criteria by two investigators (xiaoling GU and chunyan MA). Disagreements were resolved through consensus.
Data extraction
Two investigators (Xiaoling Gu and Chunyan Ma) independently extracted the required information from the final articles included. Data retrieved from the reports included the following: (I) basic information about the primary study including author, sample size, treatment strategy, time of sample collection, test method, cutoff value, (II) tumor data including histology type, disease stage, (III) results reported in the primary study including survival data, response rate, the sICAM-1 concentration before and after treatment as well as its relationship with clinico-pathological characteristics. If data from any of the above categories were not reported in the primary study, items were treated as "not reported." Authors of the primary studies were not contacted for unreported data.
Results
Study selection
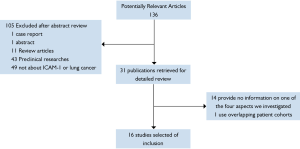
Using search strategies as described, it produced 136 potentially relevant citations between September 1992 and June 2011.Among 136 primary studies, 16 studies met the study selection section (Figure 1).In total, there were 1258 patients included, ranging from 12 to 150 patients per study. All the retained studies reported information about at least one of the four aspects investigated in this review. The major characteristics of the 16 eligible studies are listed in Tables (1,3-5. And if studies provided data for more than one of the four aspects, they would be listed more than one time.
Comparison of sICAM-1 levels between patients and controls
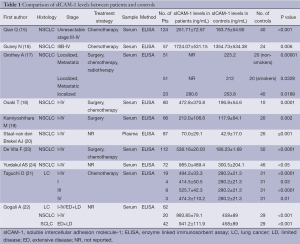
According to our literature search, 10 published studies have compared the circulating baseline sICAM-1 levels between lung cancer patients and the healthy control groups (Table 1). Although these publications followed several different patient cohorts (eight studies include various stages of lung cancer and three studies include both SCLC and NSCLC), all the ten primary studies (15-24) revealed the circulating concentration of sICAM-1 in patients with lung cancer significantly increased when compared with healthy controls (Table 1). In addition, Grothey A (17) and Taguchi O (23) both reported that the levels of sICAM-1 in patients with localized disease, even if stage I, were also significantly higher than the controls. This result apparently indicates that stimulating activities for ICAM-1 secretion starts in the early clinical stage of lung cancer.
Full table
Association between sICAM-1 levels and clinicopathological characteristics
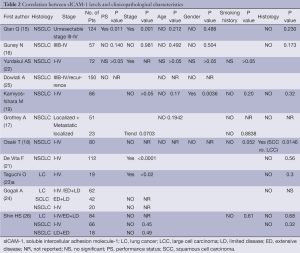
Among the 16 final articles, 11 publications examined the association between baseline sICAM-1 levels and clinico-pathological characteristics, including ECOG performance status, age, gender, smoking history, disease stage, histology type (Table 2). Most of these studies observed no significant differences in circulating sICAM-1 concentration regarding ECOG performance status (16,22,25), age (15-19,22,25), gender (15,16,18,22,25) or smoking history (17-19,22,26). However, the most recently study by Qian Q (15) including 124 unresectable NSCLC patients revealed baseline serum levels of sICAM-1 was related significantly to performance status (P=0.011). Additionally, Kamiyoshihara M (19) reported that sICAM-1 levels in male patients group was significantly higher than that in female patients group (P=0.0036), and they did not observe any significant differences between the backgrounds of males and females. However, the evidence is not powerful enough to clarify the relationship between sICAM-1 levels, performance status and gender.
Full table
10 final articles investigated the association between the baseline sICAM-1 levels and disease stages (Table 2). Both Shin HS (26) and Gogali A (24) observed no significant difference in sICAM-1 concentration among SCLC patients with different disease stages. However, of the nine studies (15-19,21,22,24,26) evaluating the relationship between sICAM-1 concentration and disease stages of NSCLC, three studies revealed a significantly positive correlation between these two variables (15,21,22). Additionally, several studies (18,19) reported that circulating sICAM-1 concentration in patients with T2、N2 disease was significantly elevated as compared with that in T1、N0 disease, respectively. Furthermore, Grothey A (17) showed that patients with metastatic diseases had markedly higher sICAM-1 levels compared with other groups (P=0.0013). Thus, there seems to be a positive correlation between the baseline sICAM-1 levels and NSCLC disease stages.
Nine studies investigated the relationship between baseline sICAM-1 levels and histological types. These studies didn't revealed any statistically difference between SCLC and NSCLC (23,24,26), as well as between adencarcinoma and squamous carcinoma (15-19,23,26). However, studies by Grothey A (17) and Kamiyoshihara M (19) observed a trend towards higher levels in squamous cell carcinomas, while Osaki T (18) reported serum sICAM-1concentration was significantly elevated in squamous cell carcinoma patients compared to large cell carcinoma patients (P=0.0146).Thus, there seems to be higher sICAM-1 levels in squamous cell carcinomas.
Changes of sICAM-1 levels before and after treatment
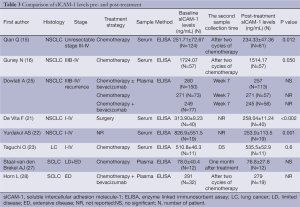
Eight studies performed pre- and post-treatment serial assessments of sICAM-1 (Table 3). Of these, two articles indicated that sICAM-1 levels in SCLC patients did not change significantly after combination chemotherapy treatment (27,28). However, four of five studies dealing with NSCLC (15,16,21,22) revealed that circulating sICAM-1 levels decreased significantly during treatment course. Additionally, as described below, there seems to be a significantly inverse association between circulating sICAM-1 levels and the response rate of advanced NSCLC. Thus the reduction of sICAM-1 levels may be related to mechanism of action of cytotoxic drugs, or to the biology of NSCLC. However, more in-depth studies are required to understand the nature of this issue.
Full table
Prognostic value of sICAM-1 levels in lung cancer
According to our literature search, 9 published studies have reported on the prognostic impact of sICAM-1levels (Table 4). And there seems to be a significantly inverse association between sICAM-1 levels and survival in NSCLC patients. However, the prognostic value of sICAM-1 in SCLC patients has not been investigated extensively.
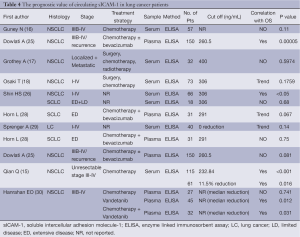
Full table
Prognostic impact of sICAM-1 on over survival
Of the 6 studies (16-18,25,26,28,29) evaluating prognostic impact on overall survival (OS), two studies revealed a significantly inverse association between baseline sICAM-1levels and OS of NSCLC patients (25,26). The study by Osaki T (18) also tended towards a poor OS at high baseline sICAM-1 levels, with a P value of 0.1759. However, another two studies (16,17) demonstrated sICAM-l obtained before treatment was not a prognostic factor concerning OS in NSCLC. This may be due to small sample size. Whereas when referred to SCLC, Horn L (28) reported that patients with lower sICAM-1 levels trended towards higher risk of death (HR=0.48; 95% CI, 0.22 to 1.02; P=0.067). And the study by Shin HS (26) didn't observe any significantly effect of sICAM-1 on OS of SCLC patients. This might reflect the difference of biology between SCLC and NSCLC.
Additionally, Sprenger A (29) demonstrated a trend towards poorer survival (8 months versus 12 months) for patients with increasing sICAM-1 levels during treatment courses, although the difference (P=0.14) did not reach significant levels.
Prognostic impact of sICAM-1 on progression free survival
Three studies evaluated the prognostic impact of pretreatment sICAM-1 levels on progression free survival (PFS). Qian Q (15) reported a significantly inverse association between sICAM-1levels and PFS of advanced NSCLC, while the study by Dowlati A (25) tended towards a poor PFS at high sICAM-1 levels (P=0.081). Whereas, Horn L (28) reported that SCLC patients with lower sICAM-1 levels showed a slight trend toward higher risk of disease progression (HR=0.75; P=0.44).
Additionally, the study by Qian Q (15) demonstrated advanced NSCLC patients who experienced more reduction of sICAM-1 levels survived significantly longer than patients who didn't. Whereas, Hanrahan EO (30) reported that the increase in sICAM-1 was associated with decreased progression risk (Table 4). These opposite results were probably due to the difference of treatment strategy as well as time of sample collection.
Predictive value of sICAM-1 levels in lung cancer
Three published studies reported the predictive value of elevated baseline sICAM-1 levels (Table 5). Of these, 2 revealed a significantly inverse association between circulating sICAM-1 levels and the response rate of advanced NSCLC (15,25), while the other study by Horn L (28) reported that baseline sICAM-1 levels did not significantly correlate with response to chemotherapy in SCLC patients. Additionally, It has to be remarked that the study by Qian Q (15) demonstrated that patients who experienced more reduction of sICAM-1 levels during the treatment courses had markedly higher response rate.

Full table
Discussion
ICAM-1 is known to participate in tumor progression and inflammatory interaction by binding with the ligands β2 integrin LFA-1(CD11a/CD18) and MAC-1(CD11b/CD18) (31). Since the soluble form of ICAM-1 (sICAM-1) was firstly detected in the serum of healthy persons in 1991, it has been investigated in a variety of malignancies. This review of the literature summarizes a portion of the large number of studies which have been published to demonstrate the clinical significance of sICAM-1 in lung cancer. And we found: (I) The circulating concentration of sICAM-1 in lung cancer patients significantly increased when compared with healthy controls; (II) Baseline sICAM-1 levels were apparently associated with clinicopathological characteristics, including ECOG performance status, gender, disease stage and histology type; (III) sICAM-1 levels seem to be able to predict outcome in patients with NSCLC.
Whereas the manner by which the soluble form of ICAM-l is generated has not been fully elucidated. Given the result of an in vitro study which revealed that sICAM-1 levels might reflect ICAM expression on cultured endothelial cells, Leeuwenberg JF (32) proposed that sICAM-1might present the extracellular part of the membrane-bound ICAM-1 which shed from the cell membrane by proteolytic cleavage. However, other studies have already identified the specific sICAM-1 mRNA transcripts in normal human bronchial epithelia cells (33). Thus sICAM-1 also can be considered as a secreted splice variant of ICAM-l lacking the intracellular and intramembrane domains. Therefore, at least two mechanisms are involved in sICAM-1 generation.
In this review, we revealed that the concentration of sICAM-1 in lung cancer patients was significantly higher when compared with that in controls. Studies by Osaki T (18) and Kamiyoshihara M (19) showed that circulating concentration of sICAM-1 in lung cancer patients was significantly correlated with T stage. Furthermore, Grothey A (17) reported there was a significant correlation between the sICAM-1 levels and the histological tumor expression of ICAM-1. All the above suggests that primary tumor cells are one of the sources of circulating sICAM-1. Additionally, Sprenger A (29) found sICAM-1 levels in patients with metastasis was higher than patients without metastasis. Therefore, they proposed that sICAM-1 also can be released by the surrounding tissues or organ metastasis. The release of sICAM-1 can be induced by various cytokines and growth factors. Lung cancer cells seem to be able to produce a variety of cytokines, and then induce the expression and secretion of ICAM-1. This may be one of the reasons for the significantly increased sICAM-1 levels in lung cancer patients.
However, the clinical and biological significance of sICAM-1in malignancy hasn't been completely understood. Springer TA (34) reported that membrane-bound ICAM-1 was a co-stimulatory factor for the T-cell receptor-mediated cellular immune response, and a shedding of ICAM-1 in tumor cells might allow themselves to escape from host anti-tumor immune response. On the other hand, circulating sICAM-1 can bind to FAL-1, and thus competitively inhibit the adhesion between leukocyte LFA-1 and ICAM-1 on tumor cells. In this way, sICAM-1 can block the interaction between tumor cells and T lymphocytes, allowing tumor cells to escape from immune surveillance (31). In addition, because increased sICAM-1level might lead to increased levels of cytokines, Shin HS (26) hypothesized that the high levels of sICAM-1 can cause tissue damage, and thus promote tumor migration and invasion. Furthermore, Gho et al. (11,12) had reported that sICAM-1 apparently has the ability to promote angiogenesis and stimulate tumor cells growth. While the study by Qian Q (15) demonstrated a significantly correlation between baseline serum levels of sICAM-1 and VEGF, and indirectly confirmed the angiogenic activity of sICAM-1 in NSCLC patients. Therefore, sICAM-1 played an important role in tumor progression and metastasis. This may also provide an explanation for the significant correction between the baseline sICAM-1 levels and disease stages.
This review revealed that three of six studies reporting on the prognostic impact of baseline sICAM-1levels in NSCLC showed us a significantly inverse association between sICAM-1levels and survival (15,25,26). While the other three negative studies were probably due to the limited patient population (16-18). Although the source of sICAM-1 has not been fully elucidated, in vitro studies using cultured endothelial cells have established that sICAM-1 levels reflect ICAM-1 expression on these cells (32). Therefore, endothelial cells seem to be an important source of sICAM-1. Furthermore, Dowlati A (25) proposed that the increased sICAM-1 levels may reflect the highly angiogenic load of tumors and result in a worse prognosis. However, neither of 2 studies evaluating the prognostic impact of sICAM-1in SCLC found a significant correlation between these two variables (26,28). It's may be a result of small sample size. But this difference might also reflect the biology of the disease.
Furthermore, both the two studies evaluating the predictive value of sICAM-1 levels revealed a significantly inverse association between circulating sICAM-1 levels and the response to chemotherapy in advanced NSCLC patients. Moreover, an in vitro study (35) observed that doxorubicin-resistant cells had markedly higher levels of adhesion molecule as compared to sensitive ones. All the above indicates that circulating sICAM-1 levels might be related to the sensitivity of tumor cells to chemotherapy. More prospective studies with a larger number of patients are called for to clarify the nature of this issue.
In conclusion, this systematic review indicates that firstly the circulating sICAM-1 levels in lung cancer patients is markedly higher than that in healthy controls. Secondly baseline sICAM-1 levels were apparently associated with performance status, gender, histology type and disease stages. Furthermore, the circulating sICAM-1 levels in NSCLC patients seems to decrease significantly due to combination chemotherapy and there seems to be a significantly inverse association between sICAM-1 levels, prognosis and response rate in non-small cell lung cancer patients. However, we should take these results with caution, as there are several limitations to this review. There is significant heterogeneity in patient cohorts (many of these studies include various stages of lung cancer and several studies include both SCLC and NSCLC) and in treatment strategy. Most of final included literatures are small sample size studies and without long term follow up. In addition, the increase of sICAM-1has been demonstrated in a great variety of benign and malignant tumors, acute and chronic diseases. Hence, additional prospective studies with a larger number of patients are required to determine the diagnostic and prognostic value of sICAM-1 in lung cancer patients.
Acknowledgements
This study was supported by a grant from the Jiangsu province Natural Science Foundation of China (BK2008326), Medical Foundation for Distinguished Scholar in Jiangsu Province (RC2007113).
Disclosure: The authors declare no conflict of interest.
References
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [PubMed]
- Liotta LA, Stracke ML. Tumor invasion and metastases: biochemical mechanisms. Cancer Treat Res 1988;40:223-38. [PubMed]
- Koyama S, Ebihara T, Fukao K. Expression of intercellular adhesion molecule 1 (ICAM-1) during the development of invasion and/or metastasis of gastric carcinoma. J Cancer Res Clin Oncol 1992;118:609-14. [PubMed]
- Johnson JP, Stade BG, Holzmann B, et al. De novo expression of intercellular-adhesion molecule 1 in melanoma correlates with increased risk of metastasis. Proc Natl Acad Sci U S A 1989;86:641-4. [PubMed]
- Witkowska AM, Borawska MH. Soluble intercellular adhesion molecule-1 (sICAM-1): an overview. Eur Cytokine Netw 2004;15:91-8. [PubMed]
- Webb DS. Mostowski =HS, Gerrard TL. Cytokine-induced enhancement of ICAM-1 expression results in increased vulnerability of tumor cells to monocyte-mediated lysis. J Immunol 1991;146:3682-6. [PubMed]
- Braakman E, Goedegebuure PS, Vreugdenhil RJ, et al. ICAM- melanoma cells are relatively resistant to CD3-mediated T-cell lysis. Int J Cancer 1990;46:475-80. [PubMed]
- Seth R, Raymond FD, Makgoba MW. Circulating ICAM-1 isoforms: diagnostic prospects for inflammatory and immune disorders. Lancet 1991;338:83-4. [PubMed]
- Berendt AR, McDowall A, Craig AG, et al. The binding site on ICAM-1 for Plasmodium falciparum-infected erythrocytes overlaps, but is distinct from, the LFA-1-binding site. Cell 1992;68:71-81. [PubMed]
- Gearing AJ, Newman W. Circulating adhesion molecules in disease. Immunol Today 1993;14:506-12. [PubMed]
- Gho YS, Kleinman HK, Sosne G. Angiogenic activity of human soluble intercellular adhesion molecule-1. Cancer Res 1999;59:5128-32. [PubMed]
- Gho YS, Kim PN, Li HC, et al. Stimulation of tumor growth by human soluble intercellular adhesion molecule-1. Cancer Res 2001;61:4253-7. [PubMed]
- Alexiou D, Karayiannakis AJ, Syrigos KN, et al. Serum levels of E-selectin, ICAM-1 and VCAM-1 in colorectal cancer patients: correlations with clinicopathological features, patient survival and tumour surgery. Eur J Cancer 2001;37:2392-7. [PubMed]
- Nakata B, Hori T, Sunami T, et al. Clinical significance of serum soluble intercellular adhesion molecule 1 in gastric cancer. Clin Cancer Res 2000;6:1175-9. [PubMed]
- Qian Q, Zhan P, Yu L, et al. Baseline levels and decrease in serum soluble intercellular adhesion molecule-1 during chemotherapy predict objective response and survival in patients who have advanced non-small-cell lung cancer. Clin Lung Cancer 2011;12:131-7. [PubMed]
- Guney N, Soydinc HO, Derin D, et al. Serum levels of intercellular adhesion molecule ICAM-1 and E-selectin in advanced stage non-small cell lung cancer. Med Oncol 2008;25:194-200. [PubMed]
- Grothey A, Heistermann P, Philippou S, et al. Serum levels of soluble intercellular adhesion molecule-1 (ICAM-1, CD54) in patients with non-small-cell lung cancer: correlation with histological expression of ICAM-1 and tumour stage. Br J Cancer 1998;77:801-7. [PubMed]
- Osaki T, Mitsudomi T, Yoshida Y, et al. Increased levels of serum intercellular adhesion molecule-1 (ICAM-1) in patients with non-small cell lung cancer. Surg Oncol 1996;5:107-13. [PubMed]
- Kamiyoshihara M, Kawashima O, Otani Y, et al. Clinical significance of the preoperative serum-soluble intercellular adhesion molecule-1 in non-small cell lung cancer. J Cardiovasc Surg (Torino) 2002;43:729-34. [PubMed]
- Staal-van den Brekel AJ, Dentener MA, Schols AM, et al. Increased resting energy expenditure and weight loss are related to a systemic inflammatory response in lung cancer patients. J Clin Oncol 1995;13:2600-5. [PubMed]
- De Vita F, Infusino S, Auriemma A, Orditura M, Catalano G. Circulating levels of soluble intercellular adhesion molecule-1 in non-small cell lung cancer patients. Oncol Rep 1998;5:393-6. [PubMed]
- Yurdakul AS, Han ER, Bukan N, Ozturk C. The Role of Serum Adhesion Molecules and Vascular Endothelial Growth Factor in Lung Cancer Patients. Turkiye Klinikleri Tip Bilimleri Dergisi 2010;30:1214-19.
- Taguchi O, Gabazza EC, Kobayashi T, et al. Circulating intercellular adhesion molecule-1 in patients with lung cancer. Intern Med 1997;36:14-8. [PubMed]
- Gogali A, Charalabopoulos K, Zampira I, et al. Soluble adhesion molecules E-cadherin, intercellular adhesion molecule-1, and E-selectin as lung cancer biomarkers. Chest 2010;138:1173-9. [PubMed]
- Dowlati A, Gray R, Sandler AB, et al. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab--an Eastern Cooperative Oncology Group Study. Clin Cancer Res 2008;14:1407-12. [PubMed]
- Shin HS, Jung CH, Park HD, et al. The relationship between the serum intercellular adhesion molecule-1 level and the prognosis of the disease in lung cancer. Korean J Intern Med 2004;19:48-52. [PubMed]
- Staal-van den Brekel AJ, Schols AM, Dentener MA, et al. The effects of treatment with chemotherapy on energy metabolism and inflammatory mediators in small-cell lung carcinoma. Br J Cancer 1997;76:1630-5. [PubMed]
- Horn L, Dahlberg SE, Sandler AB, et al. Phase II study of cisplatin plus etoposide and bevacizumab for previously untreated, extensive-stage small-cell lung cancer: Eastern Cooperative Oncology Group Study E3501. J Clin Oncol 2009;27:6006-11. [PubMed]
- Sprenger A, Schardt C, Rotsch M, et al. Soluble intercellular adhesion molecule-1 in patients with lung cancer and benign lung diseases. J Cancer Res Clin Oncol 1997;123:632-8. [PubMed]
- Hanrahan EO, Lin HY, Kim ES, et al. Distinct patterns of cytokine and angiogenic factor modulation and markers of benefit for vandetanib and/or chemotherapy in patients with non-small-cell lung cancer. J Clin Oncol 2010;28:193-201. [PubMed]
- van de Stolpe A, van der Saag PT. Intercellular adhesion molecule-1. J Mol Med (Berl) 1996;74:13-33. [PubMed]
- Leeuwenberg JF, Smeets EF, Neefjes JJ, et al. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology 1992;77:543-9. [PubMed]
- Whiteman SC, Bianco A, Knight RA, et al. Human rhinovirus selectively modulates membranous and soluble forms of its intercellular adhesion molecule-1 (ICAM-1) receptor to promote epithelial cell infectivity. J Biol Chem 2003;278:11954-61. [PubMed]
- Springer TA. Adhesion receptors of the immune system. Nature 1990;346:425-34. [PubMed]
- Rivoltini L, Cattoretti G, Arienti F, et al. The high lysability by LAK cells of colon-carcinoma cells resistant to doxorubicin is associated with a high expression of ICAM-1, LFA-3, NCA and a less-differentiated phenotype. Int J Cancer 1991;47:746-54. [PubMed]