Immunotherapy in lung cancer
Introduction
Lung cancer is the leading cause of cancer death in United States (1). Survival rates for metastatic lung cancer including non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) are poor with 5-year survival of less than 5% (1). The use of molecular targeted therapies, such as erlotinib and crizotinib, has improved median overall survival (OS) in a limited group of NSCLC patients whose tumors harbor specific genetic alterations [epidermal growth factor receptor (EGFR) sensitizing mutations: 15-18% frequency in unselected NSCLC; anaplastic lymphoma kinase (ALK) tranlocations: 2-8% frequency in unselected NSCLC] (2,3). However for a large group of NSCLC and SCLC molecular alterations are not available to lead to direct targeted therapies.
Immunotherapeutics can be defined as a broad class of therapies designated to elicit immune-mediated destruction of tumor cells. Different approaches have been undertaken to stimulate immune response against cancer such as therapeutic vaccines, immunomodulators, autologous cellular therapies, monoclonal antibodies directed against checkpoint inhibitor signals on activated T cells and/or cancer cells. Historically, immunotherapy has had minimal success in lung cancer, resulting in the common belief that lung cancer is nonimmunogenic (4). However, there are many ways in which lung cancer cells are able to evade the immune system, including secretion of immunosuppressive cytokines, loss of major histocompatibility complex antigen expression and expression of molecules that inhibit T cell activation. Recent promising results have been reported with the checkpoint inhibitors targeting cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and the programmed death-1 (PD-1) pathway with achievement of durable clinical responses with manageable toxicity also in previously heavily treated lung cancer patients. This article will review the recent clinical trials of immunotherapeutic agents in NSCLC and SCLC.
Immunotherapy in NSCLC
Vaccines for stage I-III NSCLC
Therapeutic cancer vaccination is an antigen-specific immunotherapy that primes the immune system to produce antigen-specific antibodies, CD4+ T helper cells and CD8+ cytotoxic T-lymphocytes against relevant tumor-associated antigens. Different types of immunoadjuvants are used, including phospholipid, aluminum formulation, viral vector, dendritic cells or liposome presentation. In this section we will review the different strategies of vaccination that are in the later stages of clinical development. Two vaccines (MAGE-A3 and liposomal-BLP25) are in clinical development for the treatment of potentially curable NSCLC. The others have been tested in stage IIIB-IV NSCLC (Table 1).
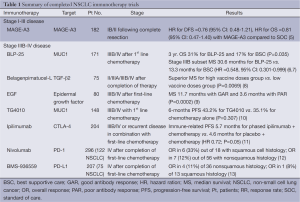
Full table
MAGE-A3 vaccine
MAGE-A3 is an antigen specifically associated with various solid tumors, including NSCLC. It is a promising target for immunotherapy because it is almost exclusively expressed on the cell surface of cancer cells. Studies indicate that 17-50% of NSCLC tumors express MAGE-A3 on their surface and expression has been associated with a poor prognosis (5,14).
The MAGE-A3 antigen-specific immunotherapeutic (ASCI) is composed of a recombinant fusion protein (MAGE-A3 and protein D of Hemophilus influenza) in combination with an immune-enhancing adjuvant system.
In a double-blind, phase II study, 182 patients with MAGE-A3-positive resected stage IB/II NSCLC were randomly assigned to vaccine or placebo in a 2:1 ratio (Table 1) (5,15). Patients received five doses of MAGE-A3 ASCI or placebo once every three weeks followed by a maximum of eight consolidation doses given once every three months. The long-term analysis showed a positive trend for MAGE-A3 treatment in this setting, with a nonsignificant but clinically relevant improvement in disease-free interval (DFI) [hazard ratio (HR) 0.75; 95% CI: 0.46-1.23; P=0.254], disease free survival (DFS) (HR 0.76; 95% CI: 0.48-1.21; P=0.248), and OS (HR 0.81; 95% CI: 0.47-1.40; P=0.454) in favor of the MAGE-A3 group. Overall treatment was very well tolerated, resulting in high therapy compliance.
Based on the experience with MAGE-A3 immunotherapy in advanced melanoma, a gene expression analysis of the tumors identified a signature of immune-related genes that appeared to be associated with a high risk for relapse. In fact, patients without this signature had a 5% risk for relapse (15). This signature has been incorporated as a secondary endpoint in the phase III MAGRIT (MAGE-A3 as adjuvant non-small-cell lung cancer immunotherapy) study (16). The MAGRIT study screened more than 13,000 patients with resected stage IB-IIIA NSCLC for immunohistochemical expression of the MAGE-A3 antigen. Ultimately, 2,270 patients were accrued by October 2011 (17). Prior adjuvant chemotherapy was not an exclusion criterion. Patients were randomly assigned 2:1 to vaccine or placebo and therapy was administered three times a week for five weeks followed by every 12 weeks for eight doses (16). DFS is the primary endpoint and prospective validation of the predictive value of the gene signature is a co-primary endpoint. Results from this study are expected in mid to late 2013.
Liposomal BLP25
MUC1 is a membrane-bound glycoprotein that becomes overexpressed and undergoes aberrant glycosylation with malignant transformation of diverse tumor types including NSCLC (18). Liposomal BLP25 (L-BLP25, Stimuvax) is a liposomal vaccine consisting of 25 amino acids from the immunogenic variable number of tandem-repeats region of MUC1 combined with an immunoadjuvant monophosphoryl lipid A in a liposomal delivery system (19). The compound was studied in a phase II multicenter randomized clinical trial with 171 patients with stable or responding stage IIIB (38%) or IV (62%) NSCLC after first-line chemotherapy or after chemoradiation (6). Patients were randomly assigned to L-BLP25 plus best supportive care (BSC) (n=88) or BSC alone (n=83) (6). Patients randomly assigned to the vaccine arm received a single low dose cyclophosphamide (300 mg/m2) infusion followed three days later by the first of eight weekly subcutaneous doses of L-BLP25. At the discretion of the investigator, patients could also receive maintenance vaccine injections once every six weeks starting six weeks after the last weekly vaccination and continuing until disease progression. The primary endpoint of the study was OS. L-BLP25 was well tolerated, with the majority of adverse events consisting of mild flu-like symptoms and no reported increase in serious adverse events (L-BLP25 plus BSC 26.1% vs. BSC 36.1%). Efficacy results suggested a nonsignificant trend toward improved OS for patients who received L-BLP25 (17.4 vs. 13 months for BSC alone, adjusted HR: 0.739; 95% CI: 0.509-1.073; P=0.112). A post hoc analysis suggested that the benefit of L-BLP25 was confined to the 65 patients with stage IIIB disease who had chemotherapy and radiation therapy (adjusted HR 0.524; 95% CI: 0.261-1.052; P=0.069) with a trend toward improved two-year survival (60% vs. 36.7% for BSC). However, it should be noted that this analysis was not a prespecified endpoint of the study. Updated analyses suggested a continued trend toward improved survival for vaccinated patients (median OS 30.6 vs. 13.3 months) and no serious long-term safety issues.
On the basis of these findings, a large international phase III START (Stimulating Targeted Antigenic Responses To NSCLC) trial randomly assigned 1,513 patients with unresectable stage IIIB NSCLC following definitive chemoradiation to L-BLP25 with BSC or placebo plus BSC (20). This study, with a primary endpoint of OS and using the phase II vaccine schedule, completed accrual in November 2011. A press release at the end of 2012 mentioned that the START trial failed to meet its primary endpoint of improved OS with L-BLP25 (25.6 vs. 22.3 months for placebo; P=0.123), although analysis of a predefined secondary endpoint did suggest that patients who received concurrent chemoradiation may have derived some benefit from the addition of the vaccine (median OS for concurrent chemoradiation followed by vaccine: 30.8 vs. 20.6 months for concurrent chemoradiation followed by placebo; P=0.016) (20).
In Asia, the smaller phase III INSPIRE study, with a design and patient population similar to that of START, began enrollment in December 2009 and is ongoing (21). In the United States, an ongoing phase II study is examining the combination of L-BLP25 with bevacizumab after chemoradiation for stage III NSCLC (22).
Vaccines for advanced NSCLC
Belagenpumatucel-L
Transforming growth factor-beta (TGF-β) is a multifunctional cytokine that works in normal and neoplastic cells to promote epithelial differentiation and inhibit cell growth (23). Elevated levels of TGF-β2 are known to be linked to immunosuppression in cancer patients and in advanced NSCLC have been associated with a more aggressive phenotype and poor survival (24). Belagenpumatucel-L is an allogeneic whole-cell vaccine derived from four irradiated NSCLC cell lines (two adenocarcinoma, one squamous, and one large cell), that have been transfected with a plasmid containing the TGF-β2 antisense trans-gene, which downregulates TGF- β2 (8). The efficacy and safety of belagenpumatucel-L was investigated in 75 patients with stage II-IV NSCLC in a phase II study. The patients received one of three dose levels of belagenpumatucel-L (1.25×107, 2.5×107, or 5×107 cells/injection) administered as an intradermal injection once monthly or once every other month (8). No significant difference in serious adverse events was noted between dose cohorts, and the majority of adverse events were attributable to disease activity apart from flu-like symptoms, which were noted in 16% of patients. A partial response rate of 15% was achieved in a subgroup of 61 patients with stage IIIB-IV disease (across all dose levels) and 59% of all enrolled patients were free from disease progression at four months. In a subgroup analysis patients with both cellular and humoral immune response to the vaccine (n=11) had improved survival compared with those (n=24) classified as immune response-negative: median 32.5 months versus 11.6 months (95% CI: 5.6-17.6; P=0.011) (25). In a subsequent phase II study that enrolled 20 patients with stage IV NSCLC, no partial or complete responses were noted. However, 14 of 20 patients had stable disease at four months and no new safety issues were noted (26). Belagenpumatucel-L was further investigated in the randomized phase III STOP trial compared to placebo as maintenance therapy after standard platinum doublet chemotherapy for stage III-IV NSCLC (27). The primary endpoint was OS and the study completed enrollment of more than 500 patients in mid-2012. Results from this study are awaited.
EGF vaccine
The EGFR pathway is integral to the growth and metastasis of NSCLC. High EGFR expression is common in NSCLC and EGFR gene mutations are associated to response to EGFR tyrosine kinase inhibitors of the internal part of this receptor (28,29). The EGF vaccine (CIMAvax EGF) was developed in Cuba and consists of human recombinant EGF combined with a Neisseria Meningitidis-derived carrier protein and an immunoadjuvant (30).
In a phase II study, 80 patients with stage IIIB-IV NSCLC previously treated with first-line platinum doublet chemotherapy, were randomized 1:1 to receive the EGF vaccine plus BSC or BSC alone (9). After priming with cyclophosphamide (200 mg/m2), patients in the vaccine group received the vaccine on day 1, 7, 14, 28 and monthly thereafter (9). A trend towards increased survival was observed in all vaccinated patients compared with controls, and the difference was statistically significant (P=0.0124) in the subgroup with age <60 years (median OS of 11.5 months for vaccinated patients compared with 5.3 months for controls). Vaccination was well tolerated, with fewer than 25% of patients experiencing adverse events and no grade 3 or 4 events were noted. Subgroup analysis pointed at a possible predictive value of humoral immune response. Patients with anti-EGF antibody titers ≥1:4,000 and at least four-times their pre-immunization values had a median OS of 11.7 months compared with 3.6 months for those without antibody response.
Building on these results, an international phase III study has completed enrollment of 579 patients with advanced NSCLC who had stable or responding disease following initial platinum doublet chemotherapy (31). Based on an exploratory analysis from the phase II study suggesting that younger patients may derive more benefit from EGF vaccination, enrollment on the phase III study was confined to patients aged 20-65 years. Efficacy results from this study are expected in late 2013. This vaccine is currently licensed in Cuba for the use in stage IIIB/IV NSCLC.
TG4010
TG4010 is a recombinant viral vector consisting of attenuated Ankara virus genetically modified to express MUC1 and interleukin-2 (IL-2) (32). IL-2 is included as immunoadjuvant because it is able to reverse the suppression of the T-cell response mediated by the cancer-associated MUC1 (33). In a phase II study, first-line cisplatin/gemcitabine chemotherapy was administered randomly with or without TG4010 to 148 untreated advanced NSCLC patients with tumors expressing MUC1 by immunohistochemistry (10). The vaccine was administered subcutaneously each week for 6 weeks and then every three weeks until disease progression with primary endpoint of six-month PFS and a target rate of 40% or higher in the experimental group. The addition of TG4010 seemed to enhance the effect of chemotherapy with a progression-free survival rate at six months of 43% (95% CI: 33-54%) in the TG4010 plus chemotherapy group and 35% (95% CI: 26-45%) in the chemotherapy alone group. Median OS was not statistically significant different between groups.
The prespecified analysis of the cellular immune response to MUC1 did not show a significant difference between vaccinated and unvaccinated patients. However, an exploratory analysis suggested that increased levels of activated natural killer (aNK) cells might inhibit response to the vaccine. In fact, the median OS was 18 months in patients with normal levels of aNK cells, while it was 11.3 months in those with high levels of aNK cells (P=0.02). The authors hypothesized that in addition to their function in tumor cell death, NK cells also inhibit the adaptive immune response if present at highly elevated levels.
In January 2012, a phase IIB/III study of TG4010 was launched, comparing first-line platinum doublet chemotherapy with TG4010 to chemotherapy alone with primary endpoint of OS for the phase III (34). In the phase IIB part, the predictive role of aNK will be evaluated based on a PFS endpoint. Patients enrolled in this study will receive weekly subcutaneous injections of vaccine or placebo during the first six weeks of chemotherapy followed by injections once every three weeks until disease progression (34).
Immune checkpoint blockade
Several checkpoint molecules exist which dampen the T-cell immune response to antigens expressed by tumor cells (35). Monoclonal antibodies interacting with two of these molecular pathways, PD-1 and CTLA-4, have shown activity in advanced NSCLC. Immune checkpoint inhibition is of particular interest because some patients with advanced solid tumors appear to derive unusually prolonged benefits; this has been most clearly demonstrated in metastatic melanoma, where approximately 5-8% of patients will live many years with stable or responsive disease after anti-CTLA-4 antibody treatment (36,37).
Anti-CTLA-4
Monoclonal antibodies against CTLA-4 are designed to prevent the interaction between CTLA-4 and its ligands (CD80/CD86) resulting in blockade of the inhibitory signal provided by CTLA-4 and subsequently enhancement of activation and proliferation of tumor specific T-cells, thereby allowing an effective immune response against the tumor (38). Extensive investigation of ipilimumab for advanced melanoma led to regulatory approval showing a survival advantage in phase III clinical trials (36,37).
In lung cancer, a large double-blind phase II study assigned 204 patients with advanced NSCLC and extensive-stage SCLC randomized patients 1:1:1 to one of three arms: standard carboplatin/paclitaxel chemotherapy with placebo, concurrent ipilimumab (four doses of ipilimumab plus paclitaxel and carboplatin followed by two doses of placebo plus paclitaxel and carboplatin), or phased ipilimumab (two doses of placebo plus paclitaxel and carboplatin followed by four doses of ipilimumab plus paclitaxel and carboplatin) (11). Treatment was administered intravenous every 3 weeks for 18 weeks. Patients with stable disease or a tumor response after four cycles of chemotherapy continued to receive infusions of ipilimumab or placebo every three months until disease progression. The dose of ipilimumab used in this study was higher than that approved for melanoma (10 vs. 3 mg/kg).
The results for NSCLC were reported separately from the SCLC cohort. The results of the SCLC patients are reported in the SCLC section of this paper review. This study was innovative in being the first reported phase II study to adopt immune-related PFS (irPFS) as its primary endpoint, defined as the time from random assignment to immune-related progression or death. In fact, the observations made in melanoma clinical trials pointed the difference of pattern of responses in immunotherapies compared to the ones to cytotoxic agents (39). With immune checkpoint inhibition, a minority of patients has initially demonstrated progressive disease by traditional Response Evaluation Criteria in Solid Tumors (RECIST) followed by a delayed response to immunotherapy and in some cases prolonged survival (40). Secondary endpoints for the phase II study of ipilimumab in NSCLC included modified World Health Organization response criteria, PFS, OS, and other immune-related response criteria.
For NSCLC, the study met its primary endpoint of improved irPFS for phased ipilimumab (HR: 0.72; P=0.05) compared with control, while interestingly this was not the case comparing concurrent ipilimumab (HR: 0.81; P=0.13) with control. The reason why one schedule of ipilimumab administration resulted in statistically significant differences, while another did not is unclear and provoking. Hypotheses include that the phased regime allows the critical temporal sequencing of chemotherapy-induced antigen release prior to ipilimumab administration or the fact that concurrent chemotherapy may lower lymphocyte numbers and thus reduce the effectiveness of ipilimumab during a crucial period. The phased ipilimimab, concurrent ipilimumab, and control arms were associated with a median irPFS of 5.7, 5.5 and 4.6 months, and a median OS of 12.2, 9.7 and 8.3 months, respectively. Subset analysis for histology revealed an improved irPFS in squamous (HR: 0.55; 95% CI: 0.27-1.12) than adenocarcinoma histology (0.82) in the phased ipilimumab. In the concurrent ipilimumab arm results were similar for different histologies.
In terms of toxicity profiles, in this trial, grade 3-4 toxicity was high at 15%, 20% and 6% for phased, concurrent schedules and control group. The main side effects included diarrhea, colitis, transaminitis, and pituitary dysfunction, all of which are previously described side effects of CTLA-4 inhibition (41). Two treatment-related deaths were reported. On the basis of the phase II results, a phase III trial is ongoing comparing with a 1:1 randomization phased ipilimumab beginning after two of a planned six cycles of carboplatin-paclitaxel followed by maintenance ipilimumab or the same schedule of chemotherapy with placebo in patients with stage IV or recurrent lung squamous cell carcinoma (42). This large trial is scheduled to complete accrual in September 2014 with a total of 920 patients and primary endpoint of OS.
Anti-PD-1 and anti-PD-L1
The programmed death receptor 1 (PD-1) is a T-cell surface receptor that is member of the B7-CD28, expressed on T cells, B cells, natural killer cells (NK), activated monocytes and dendritic cells (43). The role of PD-1 in normal human physiology is to limit autoimmunity by acting as a co-inhibitory immune checkpoint expressed on the surface of T cells and other immune cells, including tumor-infiltrating lymphocytes (44). It has two ligands: programmed death receptor ligand 1 (PD-L1/B7-H1) and 2 (PD-L2/B7-DC) (45). Several agents targeting PD-1 pathway are in clinical development, including nivolumab (BMS-936558, anti-PD1 fully human IgG4), lambrolizumab (MK-3475, anti PD1 humanized IgG4), MEDI4736 (anti-PDL1), BMS-936559 (formerly MDX-1105, anti-PDL1 fully human IgG4) and MPDL-3280 (anti-PDL1). Promising early data in NSCLC have been reported in separate phase I studies of nivolumab, lambrolizumab and MPDL-3280.
Nivolumab was investigated in a large phase I dose-escalation study in patients with refractory solid tumors. A total of 296 heavily pretreated patients with advanced NSCLC, melanoma, renal cell carcinoma, castration-resistant prostate cancer, or advanced colorectal cancer were enrolled in the trial and received dose-escalated intravenous doses of nivolumab of 1, 3, 10 mg/kg every two weeks (12). Responses were assessed after each 8-week treatment cycle using Response Evaluation Criteria in Solid Tumors (RECIST), but the study treatment could be continued in clinically stable patients beyond apparent initial disease progression until progression was confirmed. Patients received up to twelve 8-week cycles until disease progression or a complete response.
Toxicity from nivolumab, including fatigue and diarrhea, was manageable, with grade 3 or 4 toxicities experienced by 14% of patients. Of note, three patients on this early study experienced fatal pneumonitis. Because of this complication, treatment protocols now include the early use of immunosuppression and this appears to mitigate toxicity.
Encouraging efficacy signals were seen in renal cell carcinoma, melanoma, and most surprisingly, NSCLC, where 16% of patients experienced an objective response and 33% were free from tumor progression at six months (12). Responses were noted in 9 of 48 patients (18.8%) with squamous NSCLC and 11 of 73 patients (15.1%) with non-squamous NSCLC, suggesting activity in both histologic subtypes (12). Long-term data on 129 patients showed an overall response rate of 17.2% (squamous 16.7%; nonsquamous 17.6%) with a median duration of response of 18.5 months (46). Drug-related adverse events (any grade) occurred in 71% of patients with NSCLC, with grade 3-4 drug-related adverse events reported in 14%. Drug-related pneumonitis occurred in 6% of patients; 2% of these were grade 3-4, and two deaths from pneumonitis occurred in patients with NSCLC. Current suggested management algorithms for patients with suspected grade 2 pneumonitis related to anti-PD-1 involve discontinuation of the drug, prompt systemic steroid therapy, and consideration of empiric antibiotics because of the challenges involved in differentiating infective versus drug-induced pneumonitis (47). Grade 3-4 pneumonitis requires immediate discontinuation of the drug, early high dose steroid administration, and consideration of adjunctive immunosuppressant therapy such as infliximab, mycophenolate mofetil, or cyclophosphamide (47). In addition, patients may require prolonged tapering of immunosuppression to avoid recurrence of the adverse event. Nivolumab has now entered phase III clinical investigation as a single agent compared with second-line single-agent chemotherapy for advanced squamous and nonsquamous NSCLC (48,49). Expression of PD-L1 by immunohistochemical analysis of tumor cells is a possible marker of response to anti-PD-1 (12). Recently reported data using lack of PD-L1 expression using these assays does not exclude the possibility of benefit from immunomodulators (50,51).
Lambrolizumab is a humanized monoclonal immunoglobulin G4 antibody against PD1 that has shown preliminary efficacy in a phase I study in advanced refractory solid tumors. Preliminary data in NSCLC patients showed that 17 patients had received lambrolizumab at one of three dose levels (1, 3, and 10 mg/kg). Lambrolizumab was generally well tolerated. Grade 2 pneumonitis responsive to steroid therapy occurred in one patient, grade 1/2 pruritus was noted in 4 of 17 patients, and one unconfirmed partial response was reported in a patient with NSCLC (52).
The anti-PD-L1 antibody, BMS-936559, was tested in a phase I trial in advanced solid tumors and disease progression after at least one prior therapy via intravenous infusion on days 1, 15, 29 every six weeks. The study enrolled 207 patients, 75 of whom had NSCLC and 49 NSCLC patients were included in the efficacy analysis. Among the 49 NSCLC patients 5 patients (4 nonsquamous and 1 squamous cell histology) experienced an objective response and 6 patients experienced disease stabilization for at least 24 weeks (13).
Most recently, the preliminary results of another PD-L1 antibody, MPDL3280A, phase I trial were presented (51,53). MPDL3280A is an immunoglobulin G4 antibody that has been engineered to remove its antibody-dependent cell-mediated cytotoxicity function, thus theoretically avoiding the killing of tumor-directed activated T cells. A cohort of 85 patients, part of a larger phase I trial in advanced solid tumors, were treated with intravenous infusion of the study drug every three weeks for a median duration of 106 days (range, 1-450 days). Of the 85 NSCLC patients, 55% were heavily pretreated with at least three prior therapies, and the majority was represented by current or former smokers (81%). Median duration of therapy was 48 weeks and ORR was 21% in the overall population and 23% in the NSCLC cohort. 17% of responders were stable over 24 weeks. The 24-week PFS was 44% in squamous cell NSCLC and 46% in nonsquamous cell NSCLC. No dose-limiting toxicity was identified in this trial, nor was any grade 3 to 5 pneumonitis or diarrhea reported. For patients treated with the study drug, increased PDL1 expression by immunohistochemistry was associated with increased response. ORR was 46% in patients with IHC2 and IHC3 and 86% in IHC3 patients. The investigators analyzed whether smoking status predicted for a differential effect and they found that former/current smokers had an ORR of 26% (n=43) compared with 10% in never smokers (n=10) (51,53).
Currently a multi-arm phase I trial is ongoing to evaluate nivolumab in combination with cisplatin/gemcitabine, cisplatin/pemetrexed, carboplatin/paclitaxel, erlotinib (in EGFR mutation-positive nonsquamous NSCLC patients) and ipilimumab, or as a monotherapy in patients with treatment-naïve stage IIIB or IV NSCLC as maintenance therapy after chemotherapy in combination with bevacizumab (54).
Immunotherapy in SCLC
Few immunotherapy trials have been conducted in SCLC (Table 2). The BEC2/BCG (Bacillus Calmette-Guerin) vaccine was studied extensively in SCLC in the past decade based on the fact that BEC2 is a monoclonal antibody that mimics GD3, a glycosphingolipid antigen highly expressed in SCLC but rarely in normal tissue (60). When BSG was combined with GD3 three out of fourteen patients developed antibodies against GD3 (61). BEC2/BCG showed promising results in an early clinical trial in SCLC by Grant et al. (62), however a large European trial by Giaccone et al. (55) demonstrated no significant statistical difference in median OS in 515 patients with limited SCLC (16.4 months with BSC and 14.3 months with BEC2/BCG; HR =1.12; 95% CI: 0.91-1.371).

Full table
Phased Ipilimumab schedule (two cycles of chemotherapy plus placebo followed by four cycles with ipilimumab) but not concurrent ipilimumab schedule (four cycles of chemotherapy plus ipilimumab followed by two cycles with placebo) improved irPFS versus control in the extensive stage SCLC cohort of a phase II randomized double blind trial in association with carboplatin/paclitaxel chemotherapy (HR: 0.64; P=0.03) (56). Ipilimumab in association with platinum-etoposide chemotherapy versus platinum-etoposide alone is currently tested in a phase III clinical trial in patients with extensive stage SCLC to enhance T cell responses and prolong OS (58). A phase I/II clinical trial of Nivolumab alone or in combination with ipilimumab is currently accruing patients with advanced metastatic solid tumors including extensive stage SCLC after first line of chemotherapy (59).
There are many challenges in developing immunotherapy for SCLC, including high burden of disease and lack of specific target for vaccine-based treatments. In addition, response to immunotherapy requires time. In extended-disease SCLC patients have significant clinical deterioration with rapid progressive disease that may not allow time to mount an appropriate immune response. Therefore, timing and schedule of immunotherapy in relation to other therapies, in particular chemotherapy represents an important tool to allow disease control and development of immune response.
Radiation and immunotherapy
The abscopal effect is an immunologically mediated phenomenon in which radiation provides an antigen release that stimulates effector immune cells to induce tumor cell death outside of the irradiation field (63). These often impressive systemic responses have been increasingly observed among patients treated with the newest generation of checkpoint inhibitors (64,65). As such, there has been rising interest in the immune potentiating effects of anti-CTLA-4, PD-1, and PDL-1 drugs to promote the abscopal effects when used in conjunction with limited radiation, where radiation can be used to provide antigen stimulation and help prime an immune response, which could potentially improve the response rate in distant unirradiated sites of disease.
Conclusions and future perspectives
Numerous trials are underway to explore the role of immunotherapy in the treatment of lung cancer. Recent studies of novel agents, especially the checkpoint inhibitors, have shown promising preliminary results in achieving meaningful and durable treatments responses with minimal manageable toxicity. Identifying patient populations that can derive the greatest benefit to treatment with immunotherapeutic agents and determining the best time in a patient’s treatment course to administer immunotherapy remain, at the moment, the most important challenging questions to answer. Combination of chemotherapy and/or radiation therapy with immunotherapy and the timing of administration need to be further investigated. Exploration of immunotherapy after stereotactic ablative radiation therapy has recently yielded encouraging responses that will need to be further tested in clinical trials. Finally combination of the two large groups of immunotherapy, antigen-specific vaccines and immunomodulatory agents, may have synergistic effects in augmenting the anti-tumor immune response.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339-46. [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [PubMed]
- Holt GE, Podack ER, Raez LE. Immunotherapy as a strategy for the treatment of non-small-cell lung cancer. Therapy 2011;8:43-54. [PubMed]
- Vansteenkiste J, Zielinski M, Linder A, et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. J Clin Oncol 2013;31:2396-403. [PubMed]
- Butts C, Murray N, Maksymiuk A, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol 2005;23:6674-81. [PubMed]
- Butts C, Maksymiuk A, Goss G, et al. Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): phase IIB randomized, multicenter, open-label trial. J Cancer Res Clin Oncol 2011;137:1337-42. [PubMed]
- Nemunaitis J, Dillman RO, Schwarzenberger PO, et al. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol 2006;24:4721-30. [PubMed]
- Neninger Vinageras E, de la Torre A, Osorio Rodriguez M, et al. Phase II randomized controlled trial of an epidermal growth factor vaccine in advanced non-small-cell lung cancer. J Clin Oncol 2008;26:1452-8. [PubMed]
- Quoix E, Ramlau R, Westeel V, et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol 2011;12:1125-33. [PubMed]
- Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012;30:2046-54. [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [PubMed]
- Gure AO, Chua R, Williamson B, et al. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin Cancer Res 2005;11:8055-62. [PubMed]
- Ulloa-Montoya F, Louahed J, Dizier B, et al. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J Clin Oncol 2013;31:2388-95. [PubMed]
- Tyagi P, Mirakhur B. MAGRIT: the largest-ever phase III lung cancer trial aims to establish a novel tumor-specific approach to therapy. Clin Lung Cancer 2009;10:371-4. [PubMed]
- Vansteenkiste J. Novel approaches in immunotherapy for non-small-cell lung cancer. Paper presented at American Association for Cancer Research Annual Meeting; April 5, 2013. Washington, DC, 2013.
- Vlad AM, Kettel JC, Alajez NM, et al. MUC1 immunobiology: from discovery to clinical applications. Adv Immunol 2004;82:249-93. [PubMed]
- Decoster L, Wauters I, Vansteenkiste JF. Vaccination therapy for non-small-cell lung cancer: review of agents in phase III development. Ann Oncol 2012;23:1387-93. [PubMed]
- Butts CA, Socinski MA, Mitchell P, et al. START: a phase III study of L-BLP25 cancer immunotherapy for unresectable stage III non-small cell lung cancer. J Clin Oncol 2013;31:abstr 7500.
- ClinicalTrials.gov. Cancer vaccine study for stage III, unresectable, non-small cell lung cancer (NSCLC) in the Asian population (NCT01015443). Available online: http://clinicaltrials.gov
- ClinicalTrials.gov. BLP25 liposome vaccine and bevacizumab after chemotherapy and radiation therapy in treating patients with newly diagnosed stage IIIA or stage IIIB non-small cell lung cancer that cannot be removed by surgery (NCT00828009). 2013.
- Bierie B, Moses HL. TGF-beta and cancer. Cytokine Growth Factor Rev 2006;17:29-40. [PubMed]
- Malkoski SP, Haeger SM, Cleaver TG, et al. Loss of transforming growth factor beta type II receptor increases aggressive tumor behavior and reduces survival in lung adenocarcinoma and squamous cell carcinoma. Clin Cancer Res 2012;18:2173-83. [PubMed]
- Fakhrai H, Tong A, Nemunaitis J, et al. Correlation of immune responses and survival in a phase II study of belagenpumatucel-L in non-small cell lung cancer. J Clin Oncol 2009;27:abstr 3013.
- Nemunaitis J, Nemunaitis M, Senzer N, et al. Phase II trial of Belagenpumatucel-L, a TGF-beta2 antisense gene modified allogeneic tumor vaccine in advanced non small cell lung cancer (NSCLC) patients. Cancer Gene Ther 2009;16:620-4. [PubMed]
- ClinicalTrials.gov. Phase III Lucanix™ vaccine therapy in advanced non-small cell lung cancer (NSCLC) following front-line chemotherapy (NCT00676507). Available online: http://clinicaltrials.gov
- Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101:13306-11. [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [PubMed]
- Gonzalez Marinello GM, Santos ES, Raez LE. Epidermal growth factor vaccine in non-small-cell lung cancer. Expert Rev Anticancer Ther 2012;12:439-45. [PubMed]
- ClinicalTrials.gov. A randomized trial to study the safety and efficacy of EGF cancer vaccination in late-stage (IIIB/IV) non-small cell lung cancer patients (NCT01444118)Available online: http://clinicaltrials.gov
- Ramlau R, Quoix E, Rolski J, et al. A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV Non-small cell lung cancer. J Thorac Oncol 2008;3:735-44. [PubMed]
- Agrawal B, Krantz MJ, Reddish MA, et al. Cancer-associated MUC1 mucin inhibits human T-cell proliferation, which is reversible by IL-2. Nat Med 1998;4:43-9. [PubMed]
- ClinicalTrials.gov. Phase IIB/III Of TG4010 immunotherapy In patients With stage IV non-small cell lung cancer (NCT01383148). Available online: http://clinicaltrials.gov
- Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol 2006;90:51-81. [PubMed]
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [PubMed]
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [PubMed]
- Pentcheva-Hoang T, Corse E, Allison JP. Negative regulators of T-cell activation: potential targets for therapeutic intervention in cancer, autoimmune disease, and persistent infections. Immunol Rev 2009;229:67-87. [PubMed]
- Hoos A, Eggermont AM, Janetzki S, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst 2010;102:1388-97. [PubMed]
- Pennock GK, Waterfield W, Wolchok JD. Patient responses to ipilimumab, a novel immunopotentiator for metastatic melanoma: how different are these from conventional treatment responses? Am J Clin Oncol 2012;35:606-11. [PubMed]
- Di Giacomo AM, Biagioli M, Maio M. The emerging toxicity profiles of anti-CTLA-4 antibodies across clinical indications. Semin Oncol 2010;37:499-507. [PubMed]
- ClinicalTrials.gov. Trial in squamous non small cell lung cancer subjects comparing ipilimumab plus paclitaxel and carboplatin versus placebo plus paclitaxel and carboplatin (NCT01285609). Available online: http://clinicaltrials.gov
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. [PubMed]
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012;24:207-12. [PubMed]
- Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793-800. [PubMed]
- Brahmer JR, Horn L, Antonia SJ, et al. Survival and long-term follow-up of the phase I trial of nivolumab (Anti-PD-1; BMS-936558; ONO-4538) in patients (pts) with previously treated advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2013;31:abstr 8030.
- Topalian S, Sznol M, Brahmer J, et al. Nivolumab (anti-PD-1; BMS-936558; ONO-4538) in patients with advanced solid tumors: survival and long-term safety in a phase I trial. J Clin Oncol 2013;31:abstr 3002.
- ClinicalTrials.gov. Study of BMS-936558 compared to docetaxel in previously treated advanced or metastatic non-squamous NSCLC (NCT01673867). Available online: http://clinicaltrials.gov
- ClinicalTrials.gov. Study of BMS-936558 compared to docetaxel in previously treated advanced or metastatic squamouscell non-small cell lung cancer (NSCLC) (NCT01642004).
- Grosso J, Horak CE, Inzunza D, et al. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced solid tumors treated with nivolumab (anti-PD-1; BMS-936558; ONO-4538). J Clin Oncol 2013;31:abstr 3016.
- Spigel DR, Gettinger SN, Horn L, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC). J Clin Oncol 2013;31:abstr 8008.
- Patnaik A, Kang SP, Tolcher AW, et al. Phase I study of MK-3475 (anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. J Clin Oncol 2012;30:abstr 2512.
- Soria JC, Cruz C, Balheda R, et al. Clinical activity, safety and biomarkers of PD-L1 blockade in non-small cell lung cancner (NSCLC): additional analyses from a clinical study of the engineered antibody MPDL3280A (anti-PDL1). European Cancer Congress (ECCO-ESMO-ESTRO). Amsterdam, Netherland, 2013.
- ClinicalTrial.gov. Sudy of Nivolumab (BMS-936558) in combination with Gemcitabine/Cisplatin, Pemetrexed/Cisplatin, Carboplatin/Paclitaxel, Bevacizumab maintenance, erlotinib, ipilimumab or as monotherapy in firtst-line or in switch maintenance in subjects with stage IIIB/IV non-small cell lung cancer (NSCLC) (NCT01454102).
- Giaccone G, Debruyne C, Felip E, et al. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971-08971B; Silva Study). J Clin Oncol 2005;23:6854-64. [PubMed]
- Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 2013;24:75-83. [PubMed]
- ClinicalTrial.gov. A phase II trial of the addition of ipilimumab to carboplatin and etoposide chemotherapy for the first line treatment of extensive stage small cell lung cancer (ICE) (NCT01331525).
- ClinicalTrial.gov. Randomized, multicenter, double-blind, phase 3 trial comparing the efficacy of ipilimumab plus etoposide/platinum versus etoposide/platinum in subjects with newly diagnosed extensive-stage disease small cell lung cancer (ED-SCLC) (NCT01450761). Available online: http://clinicaltrials.gov
- ClinicalTrial.gov. A Phase 1/2, Open-label Study of Nivolumab Monotherapy or Nivolumab Combined With Ipilimumab in Subjects With Advanced or Metastatic Solid Tumors (NCT01928394). Available online: http://clinicaltrials.gov
- Fuentes R, Allman R, Mason MD. Ganglioside expression in lung cancer cell lines. Lung Cancer 1997;18:21-33. [PubMed]
- McCaffery M, Yao TJ, Williams L, et al. Immunization of melanoma patients with BEC2 anti-idiotypic monoclonal antibody that mimics GD3 ganglioside: enhanced immunogenicity when combined with adjuvant. Clin Cancer Res 1996;2:679-86. [PubMed]
- Grant SC, Kris MG, Houghton AN, et al. Long survival of patients with small cell lung cancer after adjuvant treatment with the anti-idiotypic antibody BEC2 plus Bacillus Calmette-Guerin. Clin Cancer Res 1999;5:1319-23. [PubMed]
- Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013;105:256-65. [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [PubMed]
- Hiniker SM, Chen DS, Reddy S, et al. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol 2012;5:404-7. [PubMed]


