Management of ground-glass opacities: should all pulmonary lesions with ground-glass opacity be surgically resected?
Introduction
Ground-glass opacity (GGO) is a radiological finding in computed tomography (CT) consisting of a hazy opacity that does not obscure the underlying bronchial structures or pulmonary vessels (1). Pure GGOs are those with no solid components, whereas part-solid GGOs contain both GGO and a solid component. Pulmonary nodules with GGO have been increasingly encountered in routine clinical practice with the increasingly widespread use of CT and the increased resolution of CT imaging. The recent positive results of the National Lung Screening Trial, which reported a 20% decrease in mortality from lung cancer as a result of low-dose CT screening for patients at high risk of developing lung cancer (2), are anticipated to support the use of CT examinations and to increase the detection of GGO lesions.
GGO can be a manifestation of a wide variety of clinical features, including malignancies and benign conditions, such as focal interstitial fibrosis, inflammation, and hemorrhage (3). However, lesions with GGO that do not disappear are often lung cancer or its precursor lesions (4). Favorable prognoses for the surgical resection of lesions with a considerable amount of GGO have been reported in several retrospective studies, in which the relapse rate was reported to be null (5-8).
Because some lesions with GGO remain unchanged for years, it is unclear whether all such lesions should be surgically resected, including those that microscopy shows to contain cancer cells. It has also not yet been established which surgical procedures are well-balanced. In this article, we review the literature on GGO, with special emphasis on management of GGO-predominant pulmonary lesions.
Pathological features of lesions with GGO
Noguchi’s classification
In 1995, Noguchi et al. reviewed 236 surgically resected small peripheral adenocarcinomas ≤2 cm in diameter and proposed a histologic classification of 6 types based on tumor growth patterns (9). Type A, localized bronchioloalveolar carcinoma (BAC), revealed the replacement of alveolar-lining epithelial cells with a relatively thin stroma. Type B was characterized by localized BAC with focal structural collapse of alveoli. Type C was characterized by localized BAC with foci of active fibroblastic proliferation. Type D (poorly differentiated adenocarcinoma), Type E (tubular adenocarcinoma) and Type F (papillary adenocarcinoma) showed compressive and expanding growth. Types A and B showed no lymph node metastasis and had a better 5-year survival rate (100%) than did Type C (75%) or Types D, E, and F (52%). According to Noguchi’s classification, GGO can be found in Type A, B and C tumors that show a replacement growth pattern along the alveolar lining cells; for example, Yang et al. reported that the proportion of GGO in each of these tumor types was 92%, 52%, and 20%, respectively (10).
New international multidisciplinary classification of lung adenocarcinoma
In 2011, the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) proposed a new international multidisciplinary classification of lung adenocarcinoma (11). The terms BAC and mixed subtype adenocarcinoma are no longer used because these terms were applied to a broad spectrum of tumors. Adenocarcinomas are classified as preinvasive lesions [including atypical adenomatous hyperplasia (AAH) and adenocarcinoma in situ (AIS)], minimally invasive adenocarcinoma (MIA), and invasive adenocarcinoma. AAH is a localized small proliferation of atypical Type II pneumocytes and/or Clara cells lining the alveolar walls and respiratory bronchioles. AIS is a small (≤3 cm) solitary adenocarcinoma with pure lepidic growth, and the complete resection of AIS achieves 100% disease-specific survival. AIS corresponds to Types A and B in Noguchi’s classification. MIA is a small (≤3 cm) solitary adenocarcinoma with a predominantly lepidic pattern and ≤5 mm invasion at the largest dimension. MIA does not invade lymphatics, blood vessels, or the pleura and contains no necrosis; therefore, complete resection achieves nearly 100% disease-specific survival. MIA roughly coincides with Type C in Noguchi’s classification. In general, lung adenocarcinomas are thought to follow a linear multistep progression whereby AAH progresses to AIS, which is followed by invasive adenocarcinoma.
To discuss the association between the radiological findings of GGO and the pathological diagnosis based on the new IASLC/ATS/ERS classification, we present the updated data from our previous study on lesions with GGO. The inclusion criteria for the study were the following: (I) a lesion diameter ≤3 cm; (II) a GGO proportion >50%; and (III) observation without treatment in the prior 6 months (12). To date, 32 of the 120 lesions were surgically resected. The histological diagnoses were AAH in 3 lesions, AIS in 12, MIA in 11, and invasive adenocarcinoma in 6.
The correlation between the changes in size and the histological types is shown in Figure 1. None of the 3 AAHs increased in size, whereas some of the tumors belonging to the types other than AAH did so. From these observations, it is impossible to determine histopathologic types by changes in lesion size.
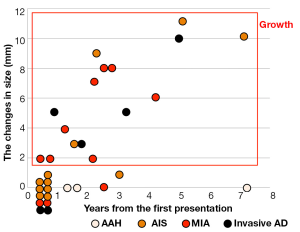
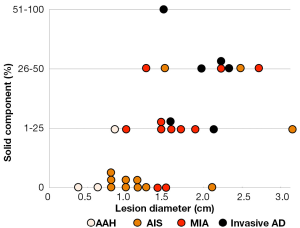
The association between the radiological findings at the time of the resection and the pathological types is shown in Figure 2. The solid component proportions were categorized as 0%, 1-25%, 26-50%, and 51-100%. Preinvasive lesions, including AAH and AIS, are typically manifested as pure GGOs, whereas more advanced adenocarcinomas may include a larger solid component within the GGO region.
Genetic features of lesions with GGO
Several reports have examined the relationship between pulmonary nodules with GGO and the relatively high frequency of epidermal growth factor receptor (EGFR) mutations. In a study of 38 patients with adenocarcinoma, the frequencies of GGO in patients with EGFR mutation and wild-types were 74% and 57%, respectively (13). In another study of 153 patients with adenocarcinoma, the GGO volume percentage in tumors with exon 21 mutation (61.7%±31.9%) was significantly higher than that in EGFR wild-type tumors (30.0%±38.5%) (14). However, the frequencies of EGFR mutation did not significantly differ (25%, 36%, 86%, to 67% in AAH, AIS, MIA, and well-differentiated adenocarcinomas, respectively) (15). Both GGO and EGFR mutations are associated with adenocarcinoma histology, female gender, and nonsmoking status.
In comparison, the incidence of KRAS mutations was 33%, 12%, 8%, and 0% in AAH, AIS, MIA, and well-differentiated adenocarcinomas, respectively, in one report (15). The overall frequency of KRAS mutations in lung adenocarcinoma was limited to 13% (16). These findings cannot be explained without assuming that some tumors with KRAS mutations might undergo regression.
The association between radiological findings of GGO and pathological invasiveness
The accuracy rate of a CT-guided core needle biopsy for nodules with GGO depends on the lesion diameter and the proportion of the GGO component; it ranges from 64.6% to 93% (17-19). Recent CT fluoroscopy-guided biopsy has a higher accuracy rate ranging from 82% to 97% (20-22). Of course, we should interpret these results in light of a possible publication bias. The article on the new IASLC/ATS/ERS classification states that AIS and MIA should not be diagnosed in small biopsies or cytology specimens and that if a noninvasive pattern is present in a small biopsy, it should be referred to as a lepidic growth pattern (11). Therefore, diagnosis usually depends on radiographic findings, which correlate closely with the pathologic diagnosis in the determination of treatment options, including surgery.
A GGO proportion of 50% or more is suggested as a cutoff value for pathological noninvasiveness in each lesion size category (Table 1) (23-28). In lesions ≤3 cm with a GGO component <50%, the rate of lymph node metastasis ranges from 10% to 26% (23-28). Based on these data, in this article, we mainly address pulmonary nodules with GGO proportion >50%.
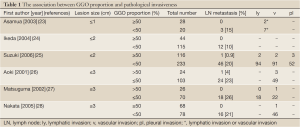
Full Table
When pathological invasiveness is defined as the presence of vascular and lymphatic invasion and lymph node metastasis, the specificity of pathological invasiveness was 100% if the cut-off value was set as a consolidation/maximum tumor diameter (C/T) ratio of ≤0.5 for lesions ≤3 cm (29). There has only been one multi-institutional prospective study to predict pathological noninvasiveness. Based on the analysis of 545 patients, Suzuki et al. reported that the specificities for the diagnosis of pathological invasiveness were 96.4% for an adenocarcinoma ≤3 cm with a C/T ratio ≤0.5 and 98.7% for an adenocarcinoma ≤2 cm with a C/T ratio ≤0.25 (30). They concluded that radiological diagnosis of noninvasive lung cancer corresponded well with pathological invasiveness, and radiological noninvasive lung adenocarcinoma could be defined as an adenocarcinoma ≤2 cm with a C/T ratio ≤0.25.
Appropriate timing for the decision to surgically resect
Because GGO-predominant lesions include malignancies, we must decide whether to resect at the first presentation. If the lesions were conservatively observed with CT examinations, we must decide when to resect them.
Recently, the Fleischner Society proposed recommendations for the management of GGOs (31). Briefly, they suggested that biopsy or surgical resection should be considered if the solid component becomes 5 mm or more.
The Japanese Society of CT Screening recommends that lesions with GGO ≥15 mm or a solid component ≥5 mm should be resected or biopsied (32).
Considering the Fleischner Society and the Japanese Society of CT Screening recommendations, we propose a conservative follow-up algorithm for pulmonary lesions ≤3 cm with a GGO component >50%, as illustrated in Figure 3.
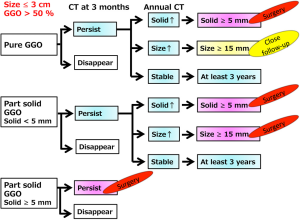
Observation with CT examinations for lesions with GGO
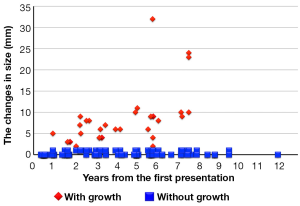
Natural history of GGO
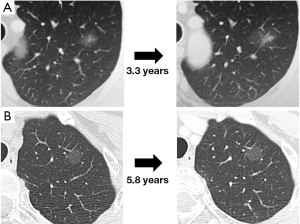
It is essential to understand the natural history of GGOs to discuss the conservative follow-up of GGO. Several reports have revealed that some lesions with GGO exhibit gradual growth, whereas others persist for years without changes (33-36). Representative CT images are presented in Figure 4. Recently, 5 reports analyzing more than 100 nodules with GGO have been published, and the results are summarized in Table 2 (12,37-40). Our study is among these reports, and our results are further illustrated in Figure 5 (12). Although the inclusion criteria and the definition of growth are variable, 10% to 27% of GGOs gradually grow, whereas others persist without changes for years (12,37-40). It should be noted that according to the updated data from our study, even some part-solid GGOs remained unchanged for more than 3 years; these included 45 pure GGOs (size range, 4 to 16 mm) and 7 part-solid GGOs (size range, 7 to 12 mm). However, the solid component proportions of these 7 part-solid GGOs were only 1-25%.
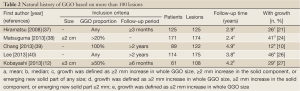
Full Table
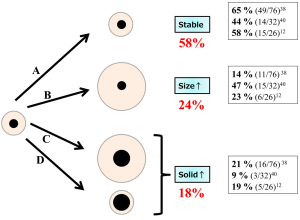
To discuss the difference between the natural history of pure GGOs and that of part-solid GGOs, we summarized them separately. Among the 5 reports mentioned above, 4 included the natural histories of pure GGOs, and these are summarized in Figure 6 (12,38-40). Approximately 80% of pure GGOs remained unchanged, while others grew in size or progressed to become part-solid GGOs. In comparison, the natural histories of part-solid GGOs were available in 3 reports; these histories are summarized in Figure 7 (12,38,40). Approximately 60% of the part-solid GGOs remained unchanged. These findings indicate that part-solid GGOs seem more likely to grow than pure GGOs are.

Volume-doubling time (VDT) of nodules with GGO
The VDT is useful for objectively evaluating GGO-predominant lesions’ tendency to grow. Based on the two-dimensional calculation method, the mean VDTs of 19 pure GGOs and 19 part-solid GGOs were 813 days (±375) and 457 days (±260), respectively (41). Other studies reported similar results: the mean VDT of pure GGOs ranged from 769 to 880 days (39,40,42). In a recent study using computer-aided three-dimensional evaluation, the mean VDTs of 19 pure GGOs and 28 part-solid GGOs were 629 (±404) days and 277 (±156) days, respectively (43). Based on these data, the VDT of pure GGOs was consistently longer than that of part-solid GGOs.
How long should we follow up nodules with GGO?
It is unclear how long we should follow GGO-predominant lesions that do not meet the criteria for surgical intervention. We analyzed the time at which lesions with GGO began to grow. Among the 108 lesions that met the abovementioned criteria, 29 lesions grew at the median follow-up period of 4.2 years. All 29 of the lesions began to grow within 3 years from the time of the first observation; of these, 13 lesions grew within 1 year, 12 lesions grew within 1.1 to 2 years, and 4 lesions grew within 2.1 to 3 years (12). Therefore, we concluded that such lesions should be followed for at least 3 years to accurately evaluate the lesion growth.
We discuss the appropriate follow-up period based on the VDT of GGO-predominant lesions. We computationally simulated the size changes of pure GGO lesions using the VDT of 813 to 880 days. A 5-mm lesion would grow to 6.7 to 6.8 mm after 3 years of observation, whereas a 10-mm lesion would grow to 13.3 to 13.6 mm within the same period (12). Are these small changes in size (i.e., 1.7 to 1.8 mm and 3.3 to 3.6 mm) detectable on CT examinations? Measurement errors should be considered when we evaluate the increase in size. Kakinuma et al. reported that increase in diameter of >1.72 mm is necessary to identify true growth, considering interobserver measurement errors (44). Therefore, these calculated changes in size should be detectable with CT analysis, and the follow up period of 3 years seems to be reasonable.
It should be noted that the range of the VDTs stated above was wide in each study, and a few lesions actually began to grow after 3 years of observation (37-39). However, it is reasonable to regard the 3-year observation follow-up period as a benchmark for GGOs because the exceptional cases are in the minority.
Surgical procedure
When the GGO lesion in question is indicated for surgical resection, the extent of surgical resection presents another question. The standard treatment for operable non-small cell lung cancer is lobectomy with dissection of the ipsilateral hilar and mediastinal lymph nodes (45). Asamura et al. reported the prognosis of 545 patients who underwent lobectomy and lymph node dissection in the abovementioned multi-institutional prospective study (30) to predict pathological noninvasiveness. At the median follow-up period of 7.1 years, with the use of the cutoff value of an adenocarcinoma ≤3 cm with a C/T ratio ≤0.5, the 5-year overall survivals of radiologic noninvasive (121 patients) and invasive (424 patients) adenocarcinomas were 96.7% and 88.9%, respectively, and the difference was statistically significant (P<0.001). With the cutoff value of an adenocarcinoma ≤2 cm with a C/T ratio ≤0.25, the 5-year overall survivals of radiologic noninvasive (35 patients) and invasive (254 patients) adenocarcinomas were 97.1% and 92.4%, respectively, and the difference was not statistically significant (P=0.259) (46). These data showed that most of the patients with adenocarcinoma ≤3 cm with a GGO component >50% were cured by lobectomy.
Based on these favorable prognoses, limited surgical resection that preserves lung parenchyma might be indicated for patients with such GGO-predominant lesions. There have been many reports on recurrence-free survival after the limited resection of a GGO lesion. For example, 35 patients with pure GGOs ≤2 cm survived without recurrence after partial resection in 31 patients and segmentectomy in 4 patients (6). Similarly, 48 patients with lesions ≤2 cm with GGO proportions >50% survived without recurrence after partial resection in 33 patients and segmentectomy in 15 patients (47).
In contrast, local recurrence has also been reported. Nakao et al. reported that 4 out of 26 patients with GGO lesions ≤2 cm developed either cut-end recurrence or metachronous primary disease more than 5 years after the initial limited resection (48). In their study, a resection margin greater than 1 cm was ensured (48). Possible reasons for the cut-end recurrence are the difficulty of intraoperatively localizing the GGO and the vague GGO border. The preoperative CT-guided injection of agar near the target GGO lesion has been reported to be useful for making deeply located lesions palpable (49). Furthermore, intraoperative ultrasonography facilitated effective localization in a completely deflated lung and was useful for evaluating surgical margins (50). This method can be performed in complete video-assisted thoracic surgery.
Regardless of the favorable prognoses that were achieved by limited resection in the retrospective studies, prospective clinical trials are necessary to establish the efficacy and safety of limited resection. There are two ongoing clinical studies in Japan to assess the efficacy of limited surgical resection for small lung cancer lesions. One study is a Phase III trial comparing lobectomy and segmentectomy for small radiologically invasive lung cancer, which is an adenocarcinoma ≤2 cm with a C/T ratio >0.25 (51). Another study is a Phase II trial of a wedge resection for small radiologically noninvasive lung cancer, which is an adenocarcinoma ≤2 cm with a C/T ratio ≤0.25 (52).
Conclusions
Surgery achieves favorable prognoses in patients with GGO-predominant lesions. However, the natural history of GGOs has been gradually clarified; some of them grow or increase their solid component, whereas others remain unchanged for years. Therefore, it remains unclear whether all GGO-predominant lesions should be surgically resected, and whether lesions without changes may not require resection. To distinguish GGOs with growth from those without growth, a 3-year observation period is a reasonable benchmark for follow-up. Future studies on the genetic differences between lesions with and without growth will help establish an appropriate management algorithm.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [PubMed]
- Park CM, Goo JM, Lee HJ, et al. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow-up. Radiographics 2007;27:391-408. [PubMed]
- Takashima S, Maruyama Y, Hasegawa M, et al. CT findings and progression of small peripheral lung neoplasms having a replacement growth pattern. AJR Am J Roentgenol 2003;180:817-26. [PubMed]
- Sawada S, Komori E, Nogami N, et al. Evaluation of lesions corresponding to ground-glass opacities that were resected after computed tomography follow-up examination. Lung Cancer 2009;65:176-9. [PubMed]
- Yamada S, Kohno T. Video-assisted thoracic surgery for pure ground-glass opacities 2 cm or less in diameter. Ann Thorac Surg 2004;77:1911-5. [PubMed]
- Mun M, Kohno T. Efficacy of thoracoscopic resection for multifocal bronchioloalveolar carcinoma showing pure ground-glass opacities of 20 mm or less in diameter. J Thorac Cardiovasc Surg 2007;134:877-82. [PubMed]
- Yoshida J, Nagai K, Yokose T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg 2005;129:991-6. [PubMed]
- Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995;75:2844-52. [PubMed]
- Yang ZG, Sone S, Takashima S, et al. High-resolution CT analysis of small peripheral lung adenocarcinomas revealed on screening helical CT. AJR Am J Roentgenol 2001;176:1399-407. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [PubMed]
- Kobayashi Y, Fukui T, Ito S, et al. How long should small lung lesions of ground-glass opacity be followed? J Thorac Oncol 2013;8:309-14. [PubMed]
- Yano M, Sasaki H, Kobayashi Y, et al. Epidermal growth factor receptor gene mutation and computed tomographic findings in peripheral pulmonary adenocarcinoma. J Thorac Oncol 2006;1:413-6. [PubMed]
- Lee HJ, Kim YT, Kang CH, et al. Epidermal growth factor receptor mutation in lung adenocarcinomas: relationship with CT characteristics and histologic subtypes. Radiology 2013;268:254-64. [PubMed]
- Sakamoto H, Shimizu J, Horio Y, et al. Disproportionate representation of KRAS gene mutation in atypical adenomatous hyperplasia, but even distribution of EGFR gene mutation from preinvasive to invasive adenocarcinomas. J Pathol 2007;212:287-94. [PubMed]
- Kosaka T, Yatabe Y, Endoh H, et al. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res 2004;64:8919-23. [PubMed]
- Shimizu K, Ikeda N, Tsuboi M, et al. Percutaneous CT-guided fine needle aspiration for lung cancer smaller than 2 cm and revealed by ground-glass opacity at CT. Lung Cancer 2006;51:173-9. [PubMed]
- Kim TJ, Lee JH, Lee CT, et al. Diagnostic accuracy of CT-guided core biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol 2008;190:234-9. [PubMed]
- Lu CH, Hsiao CH, Chang YC, et al. Percutaneous computed tomography-guided coaxial core biopsy for small pulmonary lesions with ground-glass attenuation. J Thorac Oncol 2012;7:143-50. [PubMed]
- Hur J, Lee HJ, Nam JE, et al. Diagnostic accuracy of CT fluoroscopy-guided needle aspiration biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol 2009;192:629-34. [PubMed]
- Yamauchi Y, Izumi Y, Nakatsuka S, et al. Diagnostic performance of percutaneous core needle lung biopsy under multi-CT fluoroscopic guidance for ground-glass opacity pulmonary lesions. Eur J Radiol 2011;79:e85-9. [PubMed]
- Inoue D, Gobara H, Hiraki T, et al. CT fluoroscopy-guided cutting needle biopsy of focal pure ground-glass opacity lung lesions: diagnostic yield in 83 lesions. Eur J Radiol 2012;81:354-9. [PubMed]
- Asamura H, Suzuki K, Watanabe S, et al. A clinicopathological study of resected subcentimeter lung cancers: a favorable prognosis for ground glass opacity lesions. Ann Thorac Surg 2003;76:1016-22. [PubMed]
- Ikeda N, Maeda J, Yashima K, et al. A clinicopathological study of resected adenocarcinoma 2 cm or less in diameter. Ann Thorac Surg 2004;78:1011-6. [PubMed]
- Suzuki K, Kusumoto M, Watanabe S, et al. Radiologic classification of small adenocarcinoma of the lung: radiologic-pathologic correlation and its prognostic impact. Ann Thorac Surg 2006;81:413-9. [PubMed]
- Aoki T, Tomoda Y, Watanabe H, et al. Peripheral lung adenocarcinoma: correlation of thin-section CT findings with histologic prognostic factors and survival. Radiology 2001;220:803-9. [PubMed]
- Matsuguma H, Yokoi K, Anraku M, et al. Proportion of ground-glass opacity on high-resolution computed tomography in clinical T1 N0 M0 adenocarcinoma of the lung: A predictor of lymph node metastasis. J Thorac Cardiovasc Surg 2002;124:278-84. [PubMed]
- Nakata M, Sawada S, Yamashita M, et al. Objective radiologic analysis of ground-glass opacity aimed at curative limited resection for small peripheral non-small cell lung cancer. J Thorac Cardiovasc Surg 2005;129:1226-31. [PubMed]
- Ohde Y, Nagai K, Yoshida J, et al. The proportion of consolidation to ground-glass opacity on high resolution CT is a good predictor for distinguishing the population of non-invasive peripheral adenocarcinoma. Lung Cancer 2003;42:303-10. [PubMed]
- Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751-6. [PubMed]
- Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 2013;266:304-17. [PubMed]
- Guidelines for the Management of Pulmonary Nodules Detected by Low-dose CT Lung Cancer Screening. Available online: http://www.jscts.org/pdf/guideline/gls3rd_english120719.pdf
- Kodama K, Higashiyama M, Yokouchi H, et al. Natural history of pure ground-glass opacity after long-term follow-up of more than 2 years. Ann Thorac Surg 2002;73:386-92. [PubMed]
- Kim HK, Choi YS, Kim J, et al. Management of multiple pure ground-glass opacity lesions in patients with bronchioloalveolar carcinoma. J Thorac Oncol 2010;5:206-10. [PubMed]
- Haro A, Yano T, Kohno M, et al. Ground-glass opacity lesions on computed tomography during postoperative surveillance for primary non-small cell lung cancer. Lung Cancer 2012;76:56-60. [PubMed]
- Silva M, Sverzellati N, Manna C, et al. Long-term surveillance of ground-glass nodules: evidence from the MILD trial. J Thorac Oncol 2012;7:1541-6. [PubMed]
- Hiramatsu M, Inagaki T, Inagaki T, et al. Pulmonary ground-glass opacity (GGO) lesions-large size and a history of lung cancer are risk factors for growth. J Thorac Oncol 2008;3:1245-50. [PubMed]
- Matsuguma H, Mori K, Nakahara R, et al. Characteristics of subsolid pulmonary nodules showing growth during follow-up with CT scanning. Chest 2013;143:436-43. [PubMed]
- Chang B, Hwang JH, Choi YH, et al. Natural history of pure ground-glass opacity lung nodules detected by low-dose CT scan. Chest 2013;143:172-8. [PubMed]
- Lee SW, Leem CS, Kim TJ, et al. The long-term course of ground-glass opacities detected on thin-section computed tomography. Respir Med 2013;107:904-10. [PubMed]
- Hasegawa M, Sone S, Takashima S, et al. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol 2000;73:1252-9. [PubMed]
- Aoki T, Nakata H, Watanabe H, et al. Evolution of peripheral lung adenocarcinomas: CT findings correlated with histology and tumor doubling time. AJR Am J Roentgenol 2000;174:763-8. [PubMed]
- Oda S, Awai K, Murao K, et al. Volume-doubling time of pulmonary nodules with ground glass opacity at multidetector CT: Assessment with computer-aided three-dimensional volumetry. Acad Radiol 2011;18:63-9. [PubMed]
- Kakinuma R, Ashizawa K, Kuriyama K, et al. Measurement of focal ground-glass opacity diameters on CT images: interobserver agreement in regard to identifying increases in the size of ground-glass opacities. Acad Radiol 2012;19:389-94. [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [PubMed]
- Asamura H, Hishida T, Suzuki K, et al. Radiographically determined noninvasive adenocarcinoma of the lung: survival outcomes of Japan Clinical Oncology Group 0201. J Thorac Cardiovasc Surg 2013;146:24-30. [PubMed]
- Kodama K, Higashiyama M, Takami K, et al. Treatment strategy for patients with small peripheral lung lesion(s): intermediate-term results of prospective study. Eur J Cardiothorac Surg 2008;34:1068-74. [PubMed]
- Nakao M, Yoshida J, Goto K, et al. Long-term outcomes of 50 cases of limited-resection trial for pulmonary ground-glass opacity nodules. J Thorac Oncol 2012;7:1563-6. [PubMed]
- Tsuchida M, Yamato Y, Aoki T, et al. CT-guided agar marking for localization of nonpalpable peripheral pulmonary lesions. Chest 1999;116:139-43. [PubMed]
- Kondo R, Yoshida K, Hamanaka K, et al. Intraoperative ultrasonographic localization of pulmonary ground-glass opacities. J Thorac Cardiovasc Surg 2009;138:837-42. [PubMed]
- A phase III randomised trial of lobectomy versus limited resection (segmentectomy) for small (2 cm or less) peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Available online: https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&recptno=R000002300&type=summary&language=E, accessed July 30, 2013.
- Phase II Study of Limited Surgical Resection for Peripheral Early Lung Cancer Defined with Thoracic Thin-section Computed Tomography (JCOG0804/WJOG4507L). Available online: https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&recptno=R000002262&type=summary&language=E, accessed July 30, 2013.


