Alternatives to surgery in early stage disease—stereotactic body radiotherapy
Introduction
The strictest definition of early stage non-small cell lung carcinoma (NSCLC) refers to patients with T1-2aN0 tumors (1). This chapter will focus on the management of these early stage NSCLC with radiotherapy, and specifically with high dose high precision stereotactic body radiotherapy (SBRT), also known as stereotactic ablative radiotherapy (SABR).
Currently the standard or care for early stage NSCLC is lobectomy in patients who are suitable candidates (2). However, many patients are not suitable for lobectomy due to medical co-morbidities, pulmonary function or in some circumstances patient preference. The surgical alternatives to lobectomy, in the form of sublobar resections, are being explored in such patients. Radiotherapy is an option for patients who are not able to undergo surgical resection. We do not recommend observation in this patient population, unless the patient is estimated to have an extremely limited life expectancy from comorbidities, as the median survival in patients with untreated stage I NSCLC is 14 months and the majority die of lung cancer (3). In a population based study, the introduction of SBRT lead to a reduction in the proportion of patients receiving no treatment for their early stage lung cancer, and also significantly improved the survival of patients with early stage lung cancer at the population level (4).
Prior to the widespread use of SBRT, radiotherapy involved 6 to 7 weeks of treatment with standard dose fractionation of 2 Gy per fraction daily; typical doses were 60 Gy in 30 fractions or more, to the primary tumor and surrounding lung (“involved field”) and occasionally to the lymph node regions deemed at risk of harboring microscopic disease. These regimens have the advantage of conventional dose per fraction, with potentially less late normal tissue injury (although these doses are well above radiation tolerance of lung, and some amount of lung fibrosis is to be expected), but a lower biological dose. With lower biological doses there is an expected lower rate of long-term local control (5). Clinical outcomes were generally poor with local failures occurring in approximately 40% of patients (6). The focus of therapy turned to dose escalation in the hope of improving clinical outcomes, specifically local control in this patient population.
Dose escalation strategies occurred in the form of hypofractionated regimens. Common regimens used at our institution which have acceptable efficacy, 20% local failure at 5 years, and are well tolerated are 60 Gy in 20 fractions or 50 Gy in 20 fractions (7). A Canadian national phase II study in peripheral tumors using 60 Gy in 15 fractions reported 2-year actuarial local control of 88% and 2-year overall survival of 69%. The most frequent toxicities were fatigue, cough and dyspnea. Radiation pneumonitis occurred in 10% of patients (8).
Stereotactic body radiotherapy (SBRT)
Lung SBRT or SABR involves using few high dose fractions to treat small target volume (9) guided by a set of coordinates (thus the term “stereotactic”). These coordinates are set in relationship to the precise location of the tumor, rather than a set of external marks (tattoos) or anatomical landmarks (such as bony structures), which is typical for conventional RT. The principles of body SBRT are an adaptation of the principles and experience gained from stereotactic brain RT, a well-established high-precision RT technique that uses a set of coordinates on a stereotactic frame afixed to the patient’s head, to direct multiple beams to a well-defined intracranial target. This allows the delivery of high doses of RT to the target while minimizing the exposure of normal tissue. In the case of lung cancer, the coordinates are set in relationship to the tumor itself, which can be visualized either directly with volumetric imaging such as cone-beam CT which is part of a linear accelerator, or localized through use of implanted fiducial markers, akin to what has been used with gold seed implants for prostate radiotherapy.
In addition to the use of tumor localization in the three dimensions, other important principles of stereotactic RT that need to be applied to lung SBRT are the precise outline (contouring) of a well-defined target (tumor), identification of a relatively tight (small) planning target volume (PTV) by minimizing target motion and set-up variation, conformal RT planning, using multiple small beams coming from various directions and planes, daily set-up verification prior to each treatment and the use of high RT doses that can ensure high rates of tumor cell kill.
Several single center and multicenter prospective studies, as well as numerous retrospective reports have established the safety and efficacy of lung SBRT for early stage lung cancer. There are many dose and fractionation schedules used. Local control in the order of 85-90% has been reported with most dose-fractionation schedules that provide a biologic effective dose (BED) of 100 Gy or more (10). Those schedules include 48 Gy in 4 fractions (of 12 Gy each), 55 Gy in 5 fractions (of 11 Gy each), 60 Gy in 8 fractions (of 7.5 Gy each), and 54-60 Gy in 3 fractions (of 18-20 Gy per fraction). The choice of schedule and dose depends on tumor size, location and institutional experience/preference.
In the context of lung SBRT tumors are generally <5 cm. SBRT may be considered for T1-2N0M0 and select <5 cm T3N0M0 chest wall NSCLC (11). It is our practice to deliver 54 Gy in 3 fractions for larger peripheral tumors, away from organs at risk (OAR), 48 Gy in 4 fractions for peripheral tumors <3 cm in diameter and 60 Gy in 8 fractions for centrally located tumors (i.e., tumors within a 2 cm radius of the airway or great vessels). The optimal dose for centrally located tumors is controversial and is awaiting analysis and reporting of the phase I/II RTOG study 0813 (12). In the phase II multicenter RTOG 0236 study, SBRT for early stage NSCLC in medically inoperable patients, with 60 Gy/3 fractions (equivalent to 54 Gy/3 fractions when corrected for lung tissue heterogeneity) was associated with a 3-year 98% tumor control, 91% local control and 56% overall survival (OS) (13).
Accurate mediastinal staging in potential candidates from SBRT is essential. Traditionally, patients who receive surgical resection for early stage NSCLC would have invasive mediastinal staging, either preoperative or intraoperative. In surgical patients staged preoperatively with PET/CT as N0, the occult node positivity rate at the time of surgery is 18%. Patients with tumors >3 cm or high SUVmax are at higher risk of occult nodal metastasis (14). Thus, before proceeding with SBRT, patients should at a minimum have PET staging and biopsy of any enlarged or suspicious nodes, and there may be merit in EBUS staging of other SBRT candidates who are at a high risk occult nodal disease. However, despite the absence of rigorous staging, the incidence of nodal relapse following SBRT is low, 5-10% in most series; low dose irradiation to first eschalon nodal regions has been postulated as one possible cause and immune effect of SBRT to the primary lesion in causing a presentation of antigens and resultant immune response that may control other areas of micro-metastatic disease (15), have been postulated as explanations, both have some evidence supporting them.
Technological considerations
As described above, SBRT is a technically rigorous treatment which requires precise tumor localization and treatment delivery to minimize the potential for significant toxicity to normal structures or organs at risk (OARs) (16). To accomplish this one must consider immobilization strategies, respiratory motion control, accurate target delineation, advanced planning algorithms and image guidance (17). We will briefly review the major technological considerations for the planning and delivery of SBRT focusing on motion management and image guidance.
Motion management
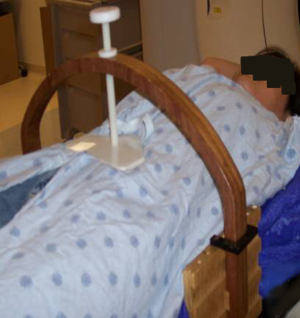
All intrathoracic tumors are affected by respiratory movement. Respiratory motion management is an essential component for the successful delivery of lung SBRT (17). There are two major strategies to manage motion in lung SBRT. The first involves reducing respiratory excursion, typically either through abdominal compression or active breathing control (ABC) (Figure 1). In some institutions tumor motion is restricted in all patients, in other institutions it is restricted in select circumstances and some institutions employ no motion restriction. When motion restriction is used selectively, a threshold is selected, commonly 1 cm (17). In our institution, using that threshold, less than 25% of patients, require abdominal compression to manage respiratory motion (17).
The second method of motion management involves using real-time tumor tracking to intermittently delivery radiotherapy when the target is in the treatment position, this is referred to as “gating”. Regardless of the technique used to manage tumor motion, accurate analysis and interpretation of the motion observed on the 4D planning CT scan and accurate localization of the tumor at the time of SBRT delivery is essential to ensure ablation of the tumor and sparing of critical structures.
Target localization
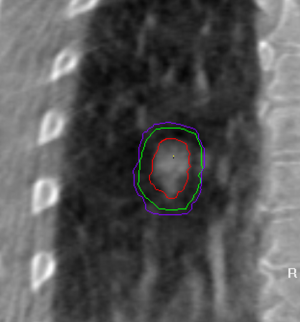
The Stereotactic Body Frame (SBF) was the immobilization strategy used in the earliest reports of extracranial SBRT (18,19) (Figure 2). Those early reports emphasized the importance of patient immobilization and accurate repositioning for multi-fraction treatments (9). Clinical outcomes with frame-based SBRT strategies were acceptable (20) however this technique requires a significant amount of treatment unit time and special equipment had to be purchased with staff trained to use it. Now, image guided strategies have been widely implemented to replace the SBF. Continued improvements in the delivery of frameless SBRT offer potential improvements in clinical outcome. Patients with poorer performance status drift more in position during SBRT (21). A change in the delivery of SBRT from multiple static beams to more contemporary volumetric modulated arc therapy (VMAT) affords a faster treatment time which may improve position accuracy by affording less time for patients to drift out of position.
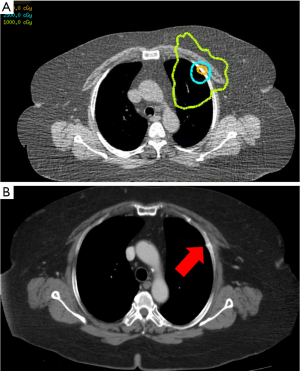
Several techniques can be used to confirm the tumor location just before or during radiotherapy. These techniques include: CT-on-rails (22), real-time tumor gating (23), TomoTherapy (24), CBCT (25), and Cyberknife (real-time tumor tracking using a robotic system) (26). The conceptual principles are as discussed above, the practical details differ depending on the system. Figure 3 demonstrates how cone beam images on the treatment unit can be used to position the patient more accurately and guide the radiation beams directly onto the tumor target.
Patient selection for SBRT
SBRT has most widely been adopted for tumors located in the periphery of the lung. In a prospective phase II study conducted by the RTOG the 3-year primary tumor control for stage I/II NSCLC treated with 18 Gy ×3 fractions was 97.6% with only 1 local failure in 55 patients. The lobar control rate at 3 years was 90.6% and the 3-year disease free survival was 48.3% (27). Overall the regimen was well tolerated with 7 patients with grade 3 toxicity and 2 patients with grade 4 toxicity. There were no grade 5 toxicities (27).
SBRT is most commonly used for patients with tumors <5 cm however some centers do deliver SBRT to larger tumors. In our experience, larger tumors still had comparable rates of local control but had higher rates of regional and distant failures, and somewhat higher rates of grade 2 pneumonitis (28).
SBRT toxicity
The rate of adverse events following SBRT is low, however in some circumstances has been severe or fatal (16). The most common side effect in the acute phase is fatigue which is typically mild (grade 1) and seen in approximately 50% of patients (11). Radiation pneumonitis can occur in the 6 weeks to 9 months following SBRT. More uncommon but worrisome due to the catastrophic nature of the outcomes are toxicities related to the central mediastinal structures such as the major vessels (aorta, vena cava etc) and the proximal airways. Rarely, grade 4 and 5 toxicities such as massive hemoptysis have been reported following SBRT, almost exclusively in the cases of central tumors (29).
Rib fractures and chest wall pain are two side-effects that are almost never reported after conventional fractionated radiotherapy, but have become widely reported and recognized to be associated with SBRT (30). Rib fractures are often asymptomatic and should not be mistaken for bone metastases (Figure 4). In a dosimetric and clinical multivariate analysis age, female gender and D0.5 were significantly associated with rib fractures following SBRT (31).
Radiation pneumonitis, a limiting toxicity with conventional RT for lung cancer, and associated with the volume of lung being treated (32) is less commonly reported in patients treated with SBRT, likely due to much smaller volumes treated, even though most patients treated with SBRT have limited lung function. One series reported that grade ≥2 pneumonitis occurred in 11% of patients (29). The risk of radiation pneumonitis is associated with increasing mean lung dose (29).
Similarly, there is minimal reduction of pulmonary function after SBRTand this treatment is suitable even for patients with severe COPD who are oxygen-dependent. At our institution we do not have a minimum cut-off for FEV1 or DLCO. All patients are considered on an individual basis for suitability for SBRT. The only group of patients who are at a higher risk of pulmonary toxicity are patients with idiopathic pulmonary fibrosis.
Radiographic changes following SBRT
The majority of patients have significant radiographic changes in their lung parenchyma following SBRT. These changes gradually develop in the 6 to 12 months following SBRT. Although the majority of patients have developed some degree of radiographic changes 12 months following SBRT the nature of these changes continue to evolve over time. There is no consensus as to how best to categorize these changes however work by Dahele et al. proposes a 4 category classification system for late post-SBRT radiographic changes. These categories are: modified conventional pattern, Mass-like fibrosis, Scar-like fibrosis and No evidence of increased density.
These radiographic changes make assessment of local control of the treated tumor following SBRT challenging. Several authors have proposed CT characteristic which may be associated with tumor recurrence as opposed to benign radiographic changes however these have not been independently validated.
The ability to accurately identify patients with residual or recurrent tumors is increasingly important as SBRT is used in operable patients where surgical salvage for a local recurrence may be an option. Further work on other imaging modalities such as MRI, perfusion CT or FLT-PET may be of clinical benefit.
Central tumors
Centrally located tumors require careful consideration when treated with SBRT. Two criteria are currently applied to identify tumors as central: the RTOG 0236 study defined them as tumors that are “within or touching the zone of the proximal bronchial tree defined as a volume 2 cm in all directions around the proximal bronchial tree (carina, right and left main bronchi, right and left upper lobe bronchi, intermedius bronchus, right middle lobe bronchus, lingular bronchus, right and left lower lobe bronchi)” (33). The RTOG 0813 trial in addition also defined as central those “tumors that are immediately adjacent to mediastinal or pericardial pleura (PTV touching the pleura)” (12). Some institutions consider central tumors to also be any tumor within 2 cm of any mediastinal structure (34) although with careful planning, avoidance of mediastinal structures should be possible in most of the latter group.
Timmerman et al. reported an excess of respiratory events in patients who received 60 Gy in 3 fractions to centrally located tumors (16). Patients with central tumors had a 2-year freedom from severe toxicity of 54%, significantly lower than patients with peripheral tumors (84%) (16). Thus lead to the introduction of modified fractionations schedules for central tumors. There is significant heterogeneity in institutional practices in that regard, and most try to achieve a BED of 100 or greater. In a patterns-of-practice survey the majority of clinicians preferred a slightly more protracted fractionation schedule (≥4 fractions) for centrally located tumors (35). It is our institutional practice to deliver 60 Gy in 8 fractions; this is supported by data from the NKI group (11,34). Other institutions have reported 50 Gy in 4 fractions (36,37), 48 Gy in 4 fractions (38), 48 Gy in 6 fractions (39), or 60 Gy in 5 fractions (39).
The RTOG phase I/II trial in patients with centrally located tumors has reached the highest planned dose level of 60 Gy in 5 fractions (12) although analysis needs to await the full one year follow-up to determine whether this is indeed the maximum tolerated dose. The hope is that this study will establish a safe and efficacious dose fractionation for central tumors and will also provide novel data on the radiation tolerance of mediastinal structures.
Medically operable patients
SBRT is now the standard of care in the majority of centers for patients who cannot have surgery for early stage NSCLC. The role of SBRT in patients who are surgical candidates remains controversial. The RTOG has completed accrual to a phase II study exploring the 2-year local control rate in medically operable patients treated with SBRT (40). A review by Onishi et al. of SBRT in medically operable patients who refused surgery reported a promising 5-year local control rate of 92% for T1 tumors and 73% for T2 tumors. The 5 year overall survival was 72% for T1 and 62% for T2 tumors (41). However, to conclusively assess the efficacy and safety of SBRT in operable patients compared to surgical resection, randomized data is needed. It is challenging to randomize patients to such different treatment modalities however, several phase III trials have been opened but all had to close due to poor accrual (42). Case-control studies that have included propensity matching (43) have demonstrated that SBRT results are at least equivalent and quite possibly superior to surgery, especially if compared to wedge resection. This is indeed intriguing and provides a solid foundation to offer SBRT even to surgical candidates.
Conclusions
SBRT is a safe and effective treatment for patients with early stage NSCLC who cannot undergo surgical resection. Further studies are needed to determine the safe standard of practice for centrally located tumors and to determine the role of SBRT in medically operable patients.
Acknowledgements
Disclosure: Drs Bezjak and Giuliani have received travel funding from Elekta Inc and Dr Bezjak has received research support from Elekta Inc in the past.
References
- Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. Chicago, IL, American Joint Committee on Cancer, 2010
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22. [PubMed]
- Raz DJ, Zell JA, Ou SH, et al. Natural history of stage I non-small cell lung cancer: implications for early detection. Chest 2007;132:193-9. [PubMed]
- Wisnivesky JP, Bonomi M, Henschke C, et al. Radiation therapy for the treatment of unresected stage I-II non-small cell lung cancer. Chest 2005;128:1461-7. [PubMed]
- Fang LC, Komaki R, Allen P, et al. Comparison of outcomes for patients with medically inoperable Stage I non-small-cell lung cancer treated with two-dimensional vs. three-dimensional radiotherapy. Int J Radiat Oncol Biol Phys 2006;66:108-16. [PubMed]
- Qiao X, Tullgren O, Lax I, et al. The role of radiotherapy in treatment of stage I non-small cell lung cancer. Lung Cancer 2003;41:1-11. [PubMed]
- Yung T, Giuliani ME, Le LW, et al. Outcomes of accelerated hypofractionated radiotherapy in stage i non-small-cell lung cancer. Curr Oncol 2012;19:e264-9. [PubMed]
- Cheung P, Faria S, Ahmed S, et al. A Phase II Study of Accelerated Hypofractionated 3-Dimensional COnfirmal Radiotherapy for Inoperable T1-3N0M0 Non-Small Cell Lung Cancer; NCIC CTG BR.25. Radiotherapy & Oncology 2012;104:S67.
- Wulf J, Hädinger U, Oppitz U, et al. Stereotactic radiotherapy of extracranial targets: CT-simulation and accuracy of treatment in the stereotactic body frame Radiother Oncol 2000;57:225-36. [PubMed]
- Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004;101:1623-31. [PubMed]
- Taremi M, Hope A, Dahele M, et al. Stereotactic body radiotherapy for medically inoperable lung cancer: prospective, single-center study of 108 consecutive patients. Int J Radiat Oncol Biol Phys 2012;82:967-73. [PubMed]
- Bezjak A. RTOG 0813: Seamless Phase I/II Study of Stereotactic Lung Radiotherapy (SBRT) for Early Stage, Centrally Located, Non-Small Cell Lung Cancer (NSCLC) in Medically Inoperable Patients. Available online: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0813
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [PubMed]
- Li L, Ren S, Zhang Y, et al. Risk factors for predicting the occult nodal metastasis in T1-2N0M0 NSCLC patients staged by PET/CT: potential value in the clinic. Lung Cancer 2013;81:213-7. [PubMed]
- Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 2009;114:589-95. [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [PubMed]
- Dahele M, Pearson S, Purdie T, et al. Practical considerations arising from the implementation of lung stereotactic body radiation therapy (SBRT) at a comprehensive cancer center. J Thorac Oncol 2008;3:1332-41. [PubMed]
- Lax I, Blomgren H, Näslund I, et al. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol 1994;33:677-83. [PubMed]
- Blomgren H, Lax I, Näslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995;34:861-70. [PubMed]
- Yoon SM, Choi EK, Lee SW, et al. Clinical results of stereotactic body frame based fractionated radiation therapy for primary or metastatic thoracic tumors. Acta Oncol 2006;45:1108-14. [PubMed]
- Li W, Purdie TG, Taremi M, et al. Effect of immobilization and performance status on intrafraction motion for stereotactic lung radiotherapy: analysis of 133 patients. Int J Radiat Oncol Biol Phys 2011;81:1568-75. [PubMed]
- Uematsu M, Shioda A, Suda A, et al. Intrafractional tumor position stability during computed tomography (CT)-guided frameless stereotactic radiation therapy for lung or liver cancers with a fusion of CT and linear accelerator (FOCAL) unit. Int J Radiat Oncol Biol Phys 2000;48:443-8. [PubMed]
- Shirato H, Shimizu S, Shimizu T, et al. Real-time tumour-tracking radiotherapy. Lancet 1999;353:1331-2. [PubMed]
- Mackie TR, Kapatoes J, Ruchala K, et al. Image guidance for precise conformal radiotherapy. Int J Radiat Oncol Biol Phys 2003;56:89-105. [PubMed]
- Jaffray DA, Drake DG, Moreau M, et al. A radiographic and tomographic imaging system integrated into a medical linear accelerator for localization of bone and soft-tissue targets. Int J Radiat Oncol Biol Phys 1999;45:773-89. [PubMed]
- Gibbs IC. Frameless image-guided intracranial and extracranial radiosurgery using the Cyberknife robotic system. Cancer Radiother 2006;10:283-7. [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [PubMed]
- Allibhai Z, Hope A, Sun A, et al. The impact of tumour size on outcomes following stereotactic body radiotherapy for medically inoperable early-stage non-small cell lung cancer. International Journal of Radiation Oncology, Biology & Physics 2013. (In Press).
- Borst GR, Ishikawa M, Nijkamp J, et al. Radiation pneumonitis in patients treated for malignant pulmonary lesions with hypofractionated radiation therapy. Radiother Oncol 2009;91:307-13. [PubMed]
- Voroney JP, Hope A, Dahele MR, et al. Chest wall pain and rib fracture after stereotactic radiotherapy for peripheral non-small cell lung cancer. J Thorac Oncol 2009;4:1035-7. [PubMed]
- Taremi M, Hope A, Lindsay P, et al. Predictors of radiotherapy induced bone injury (RIBI) after stereotactic lung radiotherapy. Radiat Oncol 2012;7:159. [PubMed]
- Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 1999;45:323-9. [PubMed]
- RTOG 0236. A Phase II Trial of Stereotactic Body Radiation Therapy (SBRT) in the Treatment of Patients with Medically Inoperable Stage I/II Non-Small Cell Lung Cancer, Available online: http://intranet.rmp.uhn.on.ca/AssetFactory.aspx?did=9471. January 2013, 2004.
- Haasbeek CJ, Lagerwaard FJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol 2011;6:2036-43. [PubMed]
- Daly ME, Perks JR, Chen AM. Patterns-of-care for thoracic stereotactic body radiotherapy among practicing radiation oncologists in the United States. J Thorac Oncol 2013;8:202-7. [PubMed]
- Chang JY, Balter PA, Dong L, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;72:967-71. [PubMed]
- Rowe BP, Boffa DJ, Wilson LD, et al. Stereotactic body radiotherapy for central lung tumors. J Thorac Oncol 2012;7:1394-9. [PubMed]
- Stauder MC, Macdonald OK, Olivier KR, et al. Early pulmonary toxicity following lung stereotactic body radiation therapy delivered in consecutive daily fractions. Radiother Oncol 2011;99:166-71. [PubMed]
- Nuyttens JJ, van der Voort van Zyp NC, Praag J, et al. Outcome of four-dimensional stereotactic radiotherapy for centrally located lung tumors. Radiother Oncol 2012;102:383-7. [PubMed]
- Timmerman RD. RTOG 0618: A Phase II Trial of Stereotactic Body Radiation Therapy (SBRT) in the Treatment of Patients with Operable Stage I/II Non-Small Cell Lung Cancer, Available online: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0618, 2010.
- Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011;81:1352-8. [PubMed]
- Hurkmans CW, Cuijpers JP, Lagerwaard FJ, et al. Recommendations for implementing stereotactic radiotherapy in peripheral stage IA non-small cell lung cancer: report from the Quality Assurance Working Party of the randomised phase III ROSEL study. Radiat Oncol 2009;4:1. [PubMed]
- Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol 2010;28:928-35. [PubMed]